Influence of Solvent, Electron Acceptors and Arenes on Photochemical Decarboxylation of Free Carboxylic Acids via Single Electron Transfer (SET)
Abstract
:1. Introduction
2. Results and Discussion
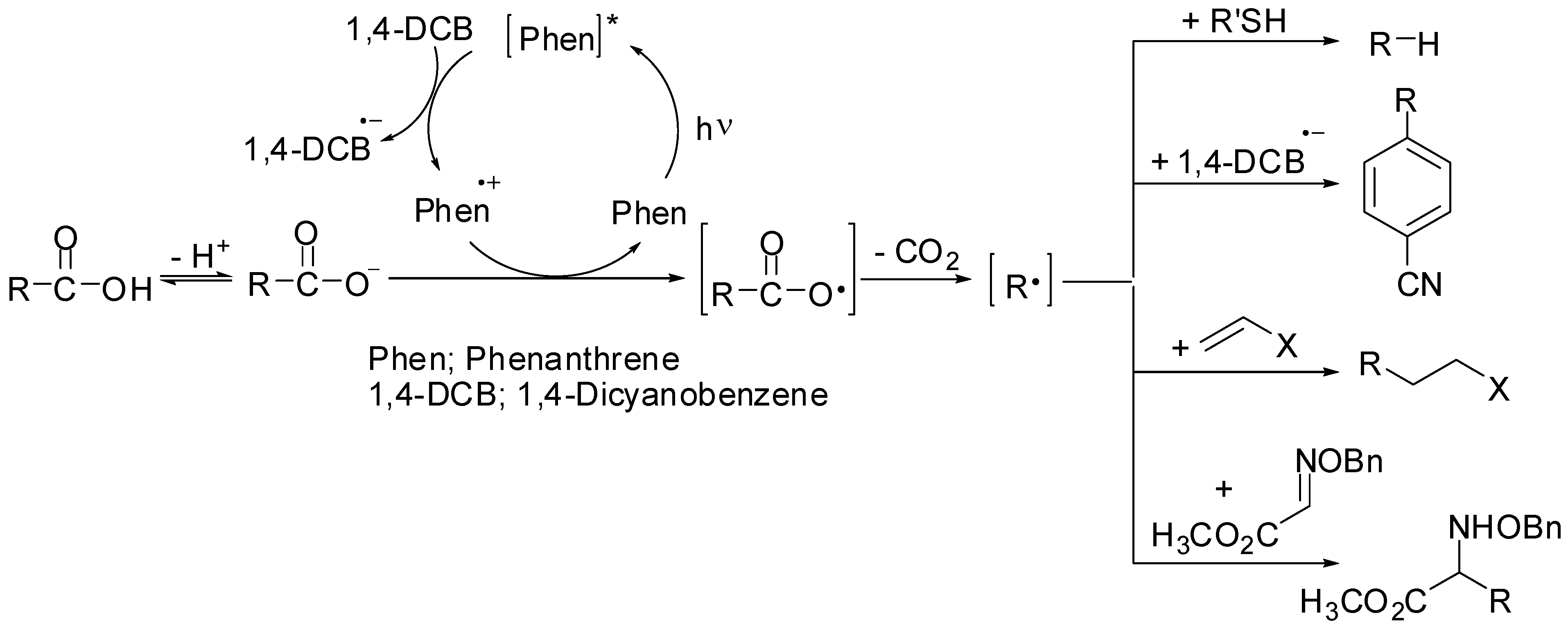
2.1. Solvent Effect
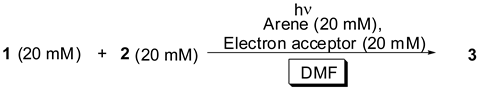
2.2. Dependence on Electron Acceptor and Arene
3. Experimental Section
3.1. General
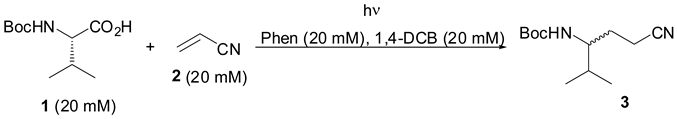
3.2. General Procedure for the Photoreaction of N-Boc-l-Valine (1) to Acrylonitrile (2): Preparation of N-tert-butoxycarbonyl-4-amino-4-isopropylbutanenitrile (3)
4. Conclusions
Acknowledgements
References and Notes
- Schaefer, H.J. Recent contributions of Kolbe electrolysis to organic synthesis. Top. Curr. Chem. 1990, 152, 91–151. [Google Scholar]
- Barton, D.H.R.; Herve, Y.; Potier, P.; Thierry, J. Manipulation of the carboxyl groups of α-amino-acids and peptides using radical chemistry based on esters of N-hydroxy-2-thiopyridone. Tetrahedron 1988, 44, 5479–5486. [Google Scholar] [CrossRef]
- Crich, D.; Quintero, L. Radical chemistry associated with the thiocarbonyl group. Chem. Rev. 1989, 89, 1413–1432. [Google Scholar] [CrossRef]
- Kurauchi, Y.; Nobuhara, H.; Ohga, K. Electron-transfer-initiated photoreactions of 1-methyl-2-phenyl-1-pyrrolinium perchlorate with α-heteroatom-substituted alkanoate anions. Bull. Chem. Soc. Jpn. 1986, 59, 897–905. [Google Scholar] [CrossRef]
- Brimage, D.R.G.; Davidson, R.S.; Steiner, P.R. Use of heterocyclic compounds as photo-sensitizers for the decarboxylation of carboxylic acids. J. Chem. Soc. Perkin Trans. I 1973, 526–529. [Google Scholar] [CrossRef]
- Oelgemoller, M.; Griesbeck, A.G. Photoinduced electron transfer chemistry of phthalimdes: an efficient tool for C-C bond formation. J. Photochem. Photobio. C 2002, 3, 109–127. [Google Scholar] [CrossRef]
- Yoon, U.C.; Jin, Y.X.; Oh, S.W.; Park, C.H.; Park, J.H.; Campana, C.F.; Cai, X.; Duesler, E.N.; Mariano, P.S. A synthetic strategy for the preparation of cyclic peptide mimetics based on SET-promoted photocyclization processes. J. Am. Chem. Soc. 2003, 125, 10664–10671. [Google Scholar] [CrossRef] [PubMed]
- Libman, J. Light induced charge transfer processes. The photochemical behavior of 1-cyanonaphthalene in the presence of phenylacetic acid derivatives. J. Am. Chem. Soc. 1975, 97, 4139–4141. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Nakao, N.; Ohashi, M. Electron-donating behavior of aliphatic carboxylic acids in the photoreaction with 1,2,4,5-tetracyanobenzene. J. Chem. Soc., Chem. Commun. 1992, 366–367. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobio. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Pac, P.; Nakasone, A.; Sakurai, H. Photosensitized electron-transfer reaction of electron donor-acceptor pairs by aromatic hydrocarbons. J. Am. Chem. Soc. 1977, 99, 5806–5808. [Google Scholar] [CrossRef]
- Majima, T.; Pac, C.; Nakasone, A.; Sakurai, H. Redox-photosensitized reactions. 7. Aromatic hydrocarbon-photosensitized electron-transfer reactions of furan, methylated furans, 1,1-diphenylethylene, and indene with p-dicyanobenzene. J. Am. Chem. Soc. 1981, 103, 4499–4508. [Google Scholar] [CrossRef]
- Ohashi, M.; Nakatani, K.; Maeda, M.; Mizuno, K. Photoinduced tandem three-component coupling of propanedinitrile, 2,5-dimethylhexa-2,4-diene, and cyanoarenes. J. Org. Chem. 2008, 73, 8348–8351. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Itou, T.; Hatanaka, M. Decarboxylative reduction of free aliphatic carboxylic acids by photogenerated cation radical. Chem. Commun. 2007, 5244–5246. [Google Scholar] [CrossRef] [PubMed]
- Itou, T.; Yoshimi, Y.; Morita, T.; Tokunaga, Y.; Hatanaka, M. Decarboxylative photosubstitution of dicyanobenzenes with aliphatic carboxylate ions. Tetrahedron 2009, 65, 263–269. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Masuda, M.; Mizunashi, T.; Nishikawa, K.; Maeda, K.; Koshida, N.; Itou, T.; Morita, T.; Hatanaka, M. Inter- and intramolecular addition reactions of electron-deficient alkenes with alkyl radicals, generated by SET-photochemical decarboxylation of carboxylic acids, serve as a mild and efficient method for the preparation of γ-amino acids and macrocyclic lactones. Org. Lett. 2009, 11, 4652–4655. [Google Scholar] [PubMed]
- Yoshimi, Y.; Kobayashi, K.; Kamakura, H.; Nishikawa, K.; Haga, Y.; Maeda, K.; Morita, T.; Itou, T.; Okada, Y.; Hatanaka, M. Addition of alkyl radicals, generated from carboxylic acids via photochemical decarboxylation, to glyoxylic oxime ether: a mild and efficient route to α-substituted α-aminoesters. Tetrahedron Lett. 2010, 51, 2332–2334. [Google Scholar] [CrossRef]
- Galicia, M.; Gonzalez, F.J. Electrochemical oxidation of tetrabutylammonium salts of aliphatic carboxylic acids in acetonitrile. J. Electrochem. Soc. 2002, 149, D46–D50. [Google Scholar] [CrossRef]
- The oxidation potentials of carboxylate ions (RCO2–) show a slight dependence on R. Thus, the value of oxidation potential for the carboxylate ion of 1 might be similar to that of the hexanoate ion: Billing, R.; Zakharova, G.V.; Atabekyan, L.S.; Hennig, H. Luminescence quenching of [UO2F2]2- in aqueous solution by anions. J. Photochem. Photobio. A 1991, 59, 163–174.
- Murov, S.L.; Carmichael, I.; Hug, G.L. “Handbook of Photochemistry”; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Tsuji, M.; Higashiyama, K.; Yamauchi, T.; Kubo, H.; Ohmiya, S. Photosubstitution reaction of cyanoaromatics with aliphatic amides. Heterocycles 2001, 54, 1027–1032. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Entry | Solvent | Yield of 3 / %a |
| 1 | CH3CN/H2O = 9:1 | 85 |
| 2b | 77 | |
| 3c | 60 | |
| 4 | CH3CN | 53 |
| 5 | DMF | 68 |
| 6 | CH3OH | 32 |
| 7 | DMSO | 13 |
| 8 | 1,4-Dioxane | 0 |
| 9 | THF | 0 |
| 10 | Benzene | 0 |

| Entry | Arene | Oxidation potential of arene / Va | Electron acceptor | Yield of 3 / %b |
| 1 | Phenanthrene | + 1.50 | 1,2-DCB | 73 |
| 2 | 1,3-DCB | 80 | ||
| 3 | Methyl 4-cyanobenzoate | 64 | ||
| 4 | 1,4-DCN | 82 | ||
| 5 | Naphthalene | + 1.70 | 1,4-DCB | 77 |
| 6 | 1-Methylnaphthalene | + 1.43 | 73 | |
| 7 | Biphenyl | 87 | ||
| 8 | Pyrene | + 1.16 | 0 | |
| 9 | Naphthalene | 1,4-DCN | 75 | |
| 10 | 1-Methylnaphthalene | 80 | ||
| 11 | Biphenyl | 93 | ||
| 12 | Pyrene | 0 |
| Entry | Arene | Oxidation potential of arene / Va | Electron acceptor | Yield of 3 / %b |
| 1 | Triphenylene | + 1.55 | 1,4-DCB | 44 |
| 2 | Chrysene | + 1.35 | 80 | |
| 3 | Naphthalene | 71 | ||
| 4 | 1-Methylnaphthalene | 68 | ||
| 5 | Biphenyl | 7 | ||
| 6 | Pyrene | 27 | ||
| 7 | Triphenylene | 1,4-DCN | 19 | |
| 8 | Chrysene | 74 | ||
| 9 | Naphthalene | 10 | ||
| 10 | 1-Methylnaphthalene | 14 | ||
| 11 | Biphenyl | 6 | ||
| 12 | Pyrene | 22 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yoshimi, Y.; Hayashi, S.; Nishikawa, K.; Haga, Y.; Maeda, K.; Morita, T.; Itou, T.; Okada, Y.; Ichinose, N.; Hatanaka, M. Influence of Solvent, Electron Acceptors and Arenes on Photochemical Decarboxylation of Free Carboxylic Acids via Single Electron Transfer (SET). Molecules 2010, 15, 2623-2630. https://doi.org/10.3390/molecules15042623
Yoshimi Y, Hayashi S, Nishikawa K, Haga Y, Maeda K, Morita T, Itou T, Okada Y, Ichinose N, Hatanaka M. Influence of Solvent, Electron Acceptors and Arenes on Photochemical Decarboxylation of Free Carboxylic Acids via Single Electron Transfer (SET). Molecules. 2010; 15(4):2623-2630. https://doi.org/10.3390/molecules15042623
Chicago/Turabian StyleYoshimi, Yasuharu, Shota Hayashi, Keisuke Nishikawa, Yoshiki Haga, Kousuke Maeda, Toshio Morita, Tatsuya Itou, Yutaka Okada, Nobuyuki Ichinose, and Minoru Hatanaka. 2010. "Influence of Solvent, Electron Acceptors and Arenes on Photochemical Decarboxylation of Free Carboxylic Acids via Single Electron Transfer (SET)" Molecules 15, no. 4: 2623-2630. https://doi.org/10.3390/molecules15042623





