Synthesis and Structure-Activity Relationships of Fenbufen Amide Analogs
Abstract
:1. Introduction
2. Results and Discussion
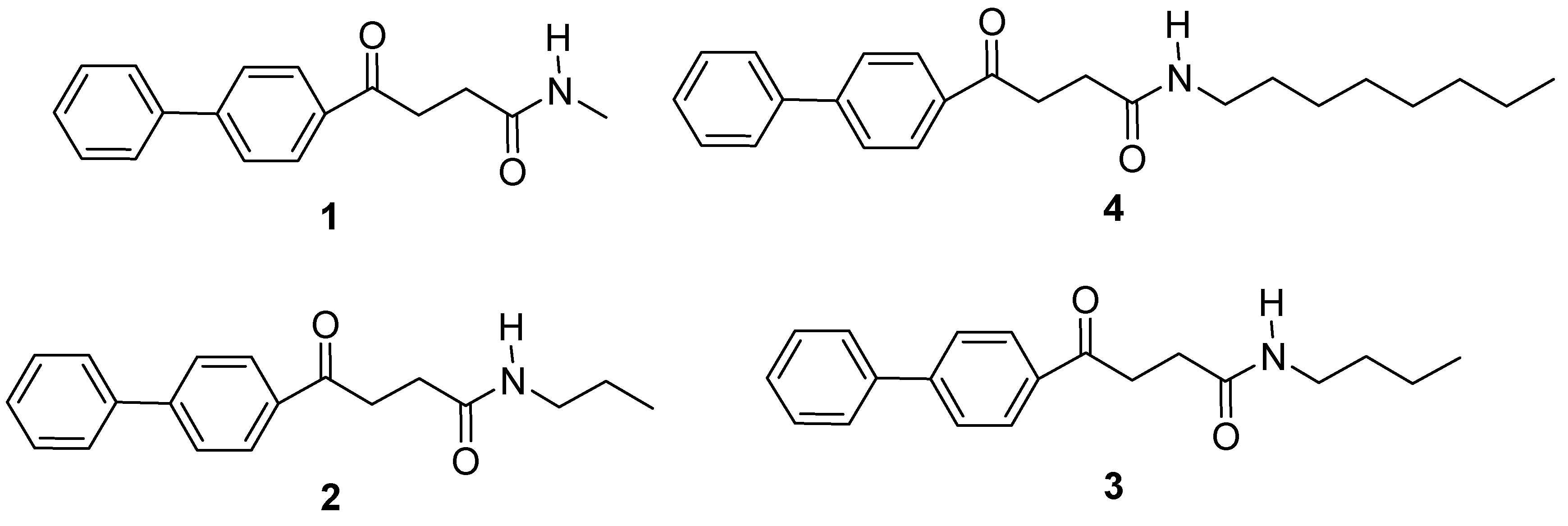
2.1. Synthesis of the fenbufen amide analogs
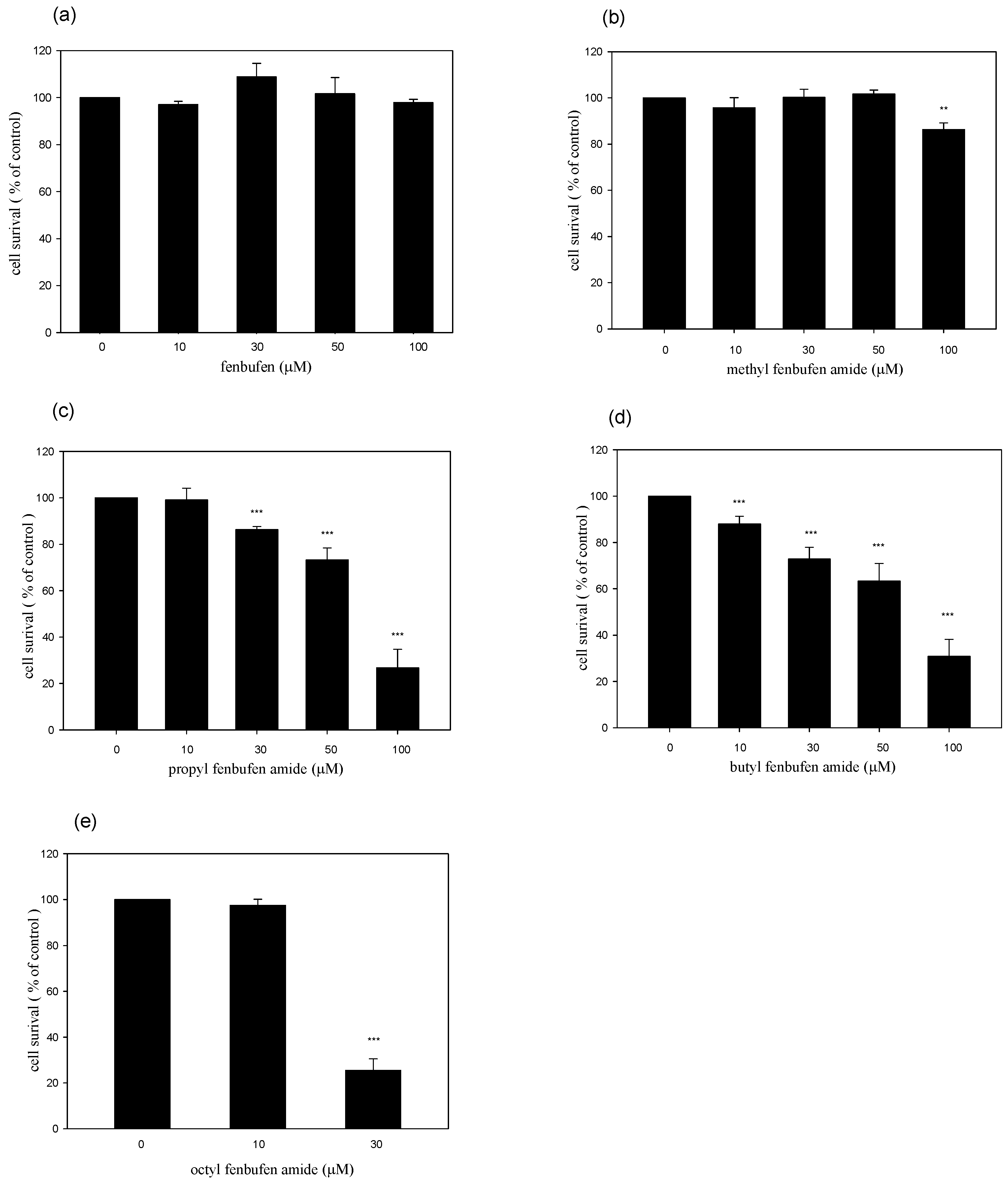
2.2. Effects of fenbufen amide analogs on cell viability
2.3. Effects of fenbufen amide analogs on NO production in LPS-activated RAW 264.7 cells
3. Experimental
3.1. General
3.2. Typical procedure for the coupling
3.3. Biological assay chemicals and reagents
3.4. Cell culture
3.5. Cell viability assay
3.6. Determination of NO production
3.7. Statistical analysis
4. Conclusions
References
- Brik, A.; Wu, C.Y.; Wong, C.H. Microtiter plate based chemistry and in situ screening: a useful approach for rapid inhibitor discovery. Org. Biomol. Chem. 2006, 4, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Best, M.; Brik, A.; Chapman, E.; Lee, L.; Cheng, W.C.; Wong, C.H. Rapid discovery of potent sulfotransferase inhibitors by diversity-oriented reaction in microplates followed by in situ screening. Chem. Biol. Chem. 2004, 5, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chang, C.F.; Chen, J.S.Y.; Lee, S.T.; Wong, C.H.; Lin, C.H. Rapid diversity-oriented synthesis in microtiter plates for in situ screening: discovery of potent and selective fucosidase inhibitors. Angew. Chem. Int. Ed. 2003, 42, 4661–4664. [Google Scholar] [CrossRef] [PubMed]
- Brik, A.; Lin, Y.C.; Elder, J.; Wong, C.H. A quick diversity-oriented amide-forming reaction to optimize P-subsite residues of HIV protease inhibitors. Chem. Biol. 2002, 9, 891–896. [Google Scholar] [CrossRef]
- Chiang, L.W.; Pei, K.; Chen, S.W.; Huang, H.L.; Lin, K.J.; Yen, T.C.; Yu, C.S. Combining a solution-phase derived library with in-situ cellular bioassay: Prompt screening of amide-forming minilibraries using MTT assay. Chem. Pharm. Bull. 2009, 57, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Kim, C.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B. 2010, 11, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Ham, Y.M.; Yoo, B.S.; Moon, J.Y.; Koh, J.; Hyun, C.G. Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. J. Biosci. Bioeng. 2009, 107, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Gaspirc, B.; Masera, A.; Skaleric, U. Immunolocalization of inducible nitric oxide synthase in localized juvenile periodontitis patients. Connect. Tissue Res. 2002, 43, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Abies koreana essential oil inhibits drug-resistant skin pathogen growth and LPS-induced inflammatory effects of murine macrophage. Lipids 2009, 44, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Chen, Y.M.; Wang, T.M.; Lee, C.K.; Chen, K.J.; Lee, T.H. Flavonoids with iNOS inhibitory activity from Pogonatherum crinitum. J. Ethnopharmacol. 2008, 118, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.W.; Pei, K.; Chen, S.W.; Huang, H.L.; Lin, K.J.; Yen, T.C.; Yu, C.S. Combining a solution-phase derived library with in-situ cellular bioassay: Prompt screening of amide-forming minilibraries using MTT assay. Chem. Pharm. Bull. 2009, 57, 714. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the corresponding author C.-S.Y. |

© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, K.-I.; Yang, C.-H.; Huang, C.-W.; Jian, J.-Y.; Huang, Y.-C.; Yu, C.-S. Synthesis and Structure-Activity Relationships of Fenbufen Amide Analogs. Molecules 2010, 15, 8796-8803. https://doi.org/10.3390/molecules15128796
Lin K-I, Yang C-H, Huang C-W, Jian J-Y, Huang Y-C, Yu C-S. Synthesis and Structure-Activity Relationships of Fenbufen Amide Analogs. Molecules. 2010; 15(12):8796-8803. https://doi.org/10.3390/molecules15128796
Chicago/Turabian StyleLin, Kun-I, Chao-Hsun Yang, Chia-Wen Huang, Jhen-Yi Jian, Yu-Chun Huang, and Chung-Shan Yu. 2010. "Synthesis and Structure-Activity Relationships of Fenbufen Amide Analogs" Molecules 15, no. 12: 8796-8803. https://doi.org/10.3390/molecules15128796




