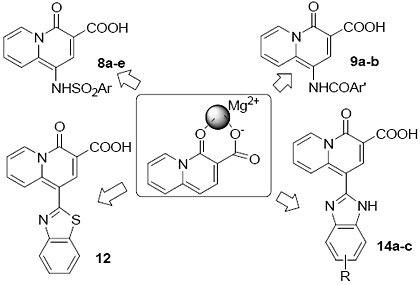
Design, Synthesis and Anti-HIV Integrase Evaluation of 4-Oxo-4H-quinolizine-3-carboxylic Acid Derivatives
Abstract
:Introduction
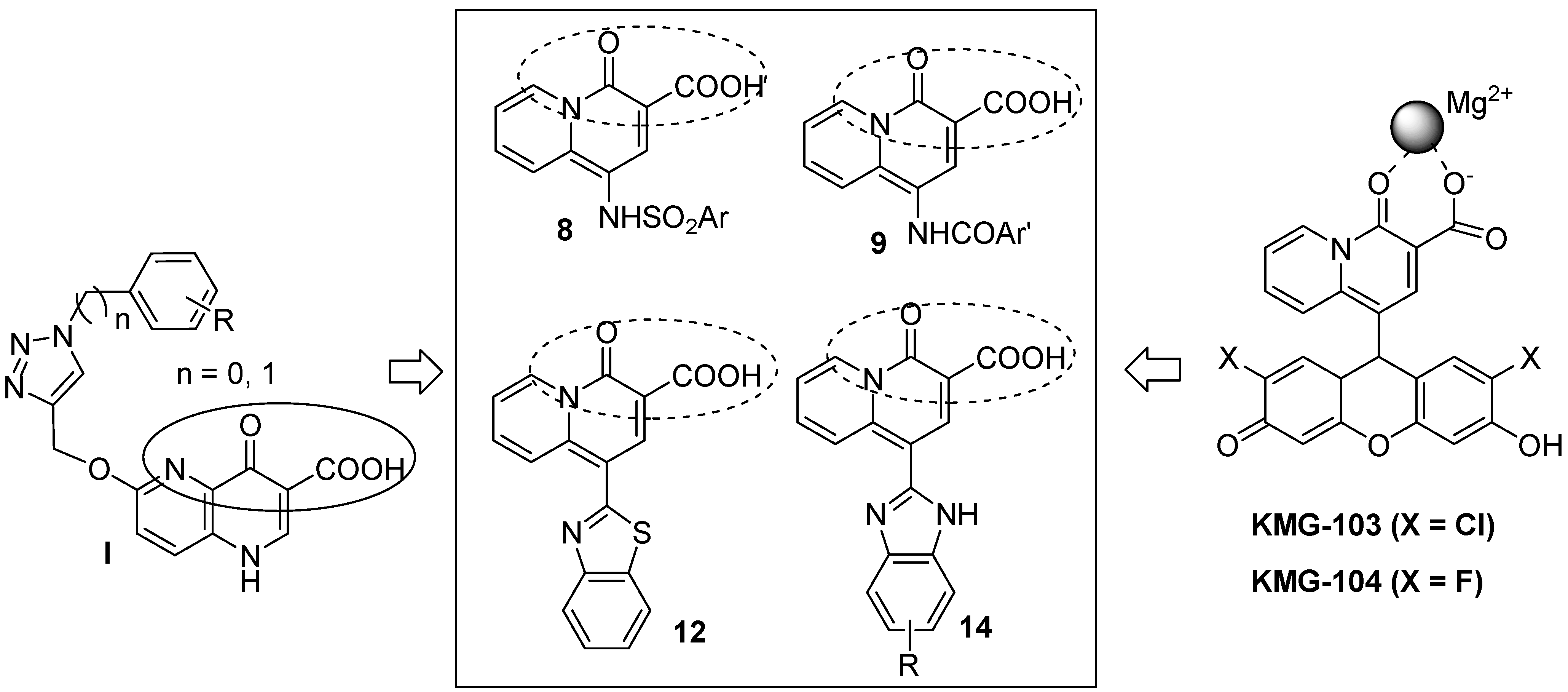
Results and Discussion
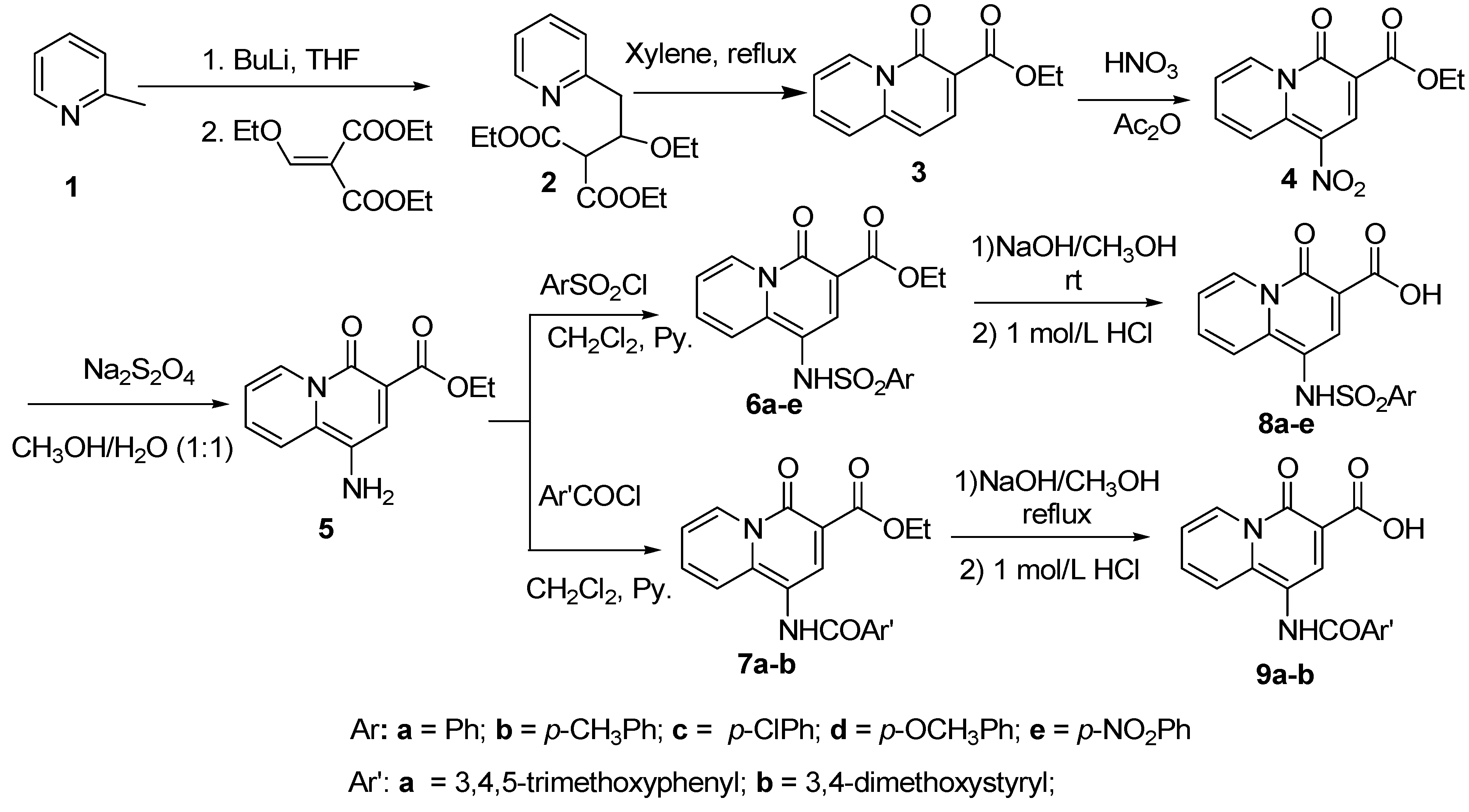
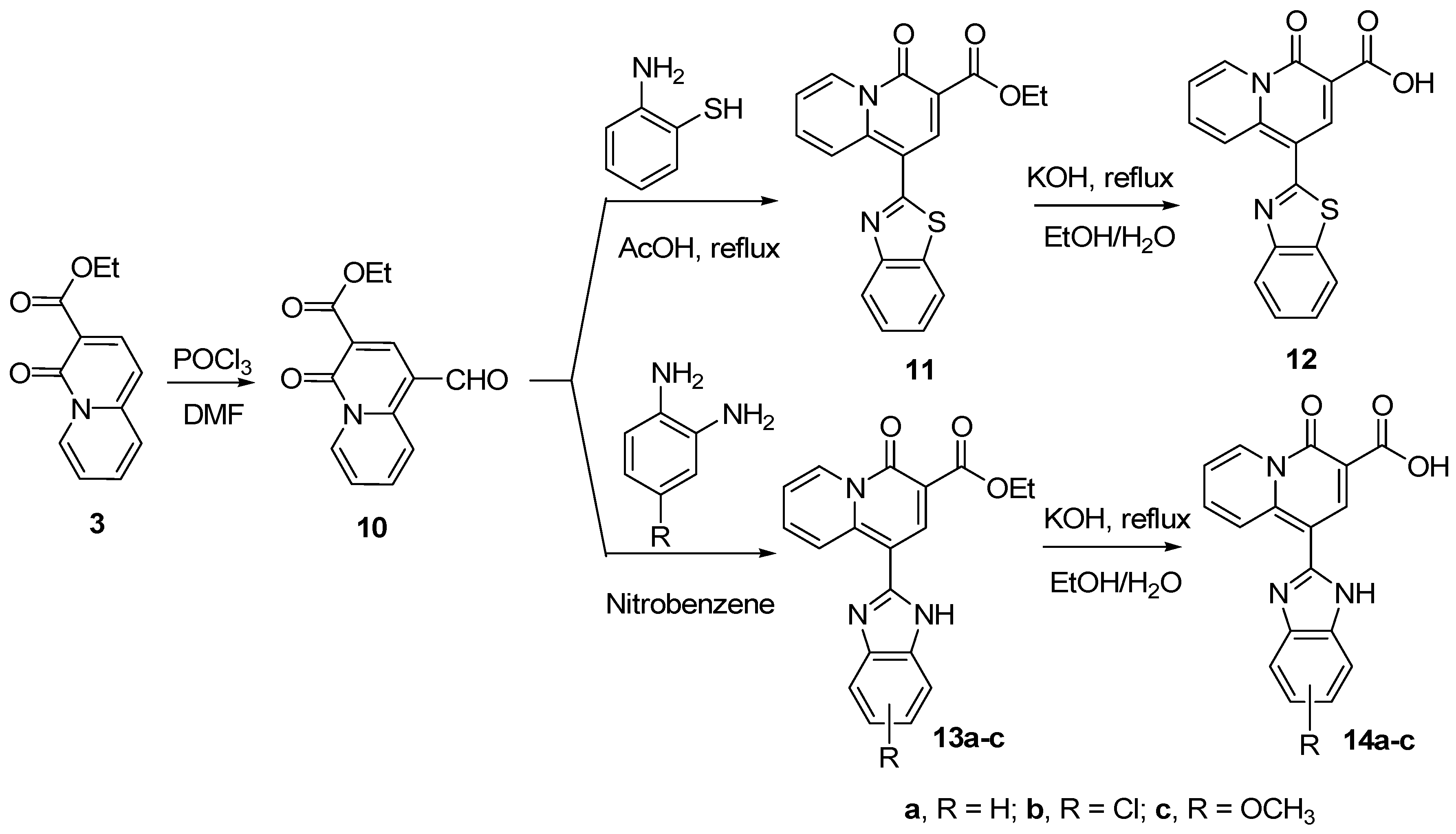
Synthesis of 4-oxo-4H-quinolizine-3-carboxylic acid derivatives 8, 9, 12 and 14
Structural analysis
Preliminary investigation of Mg2+ binding
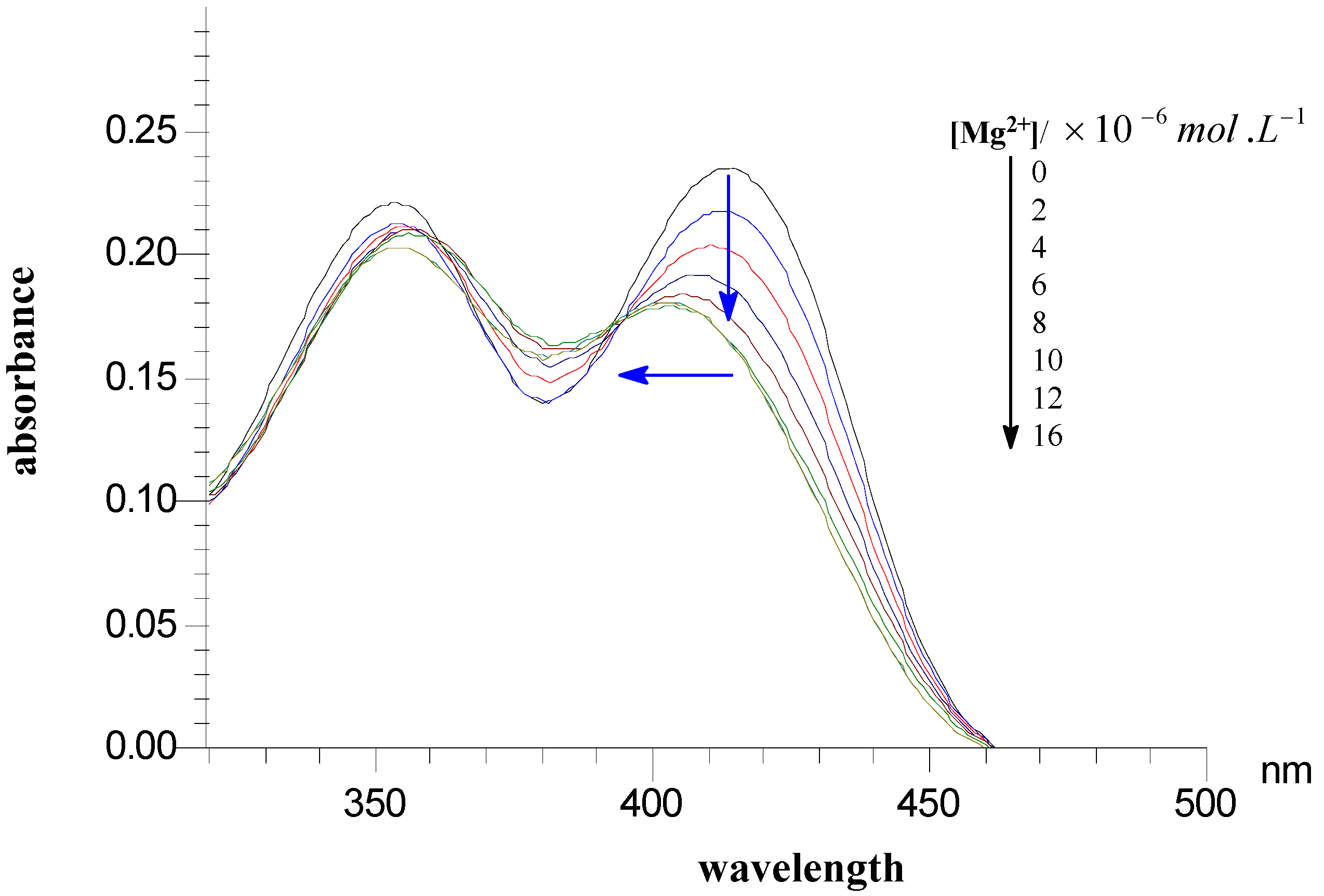
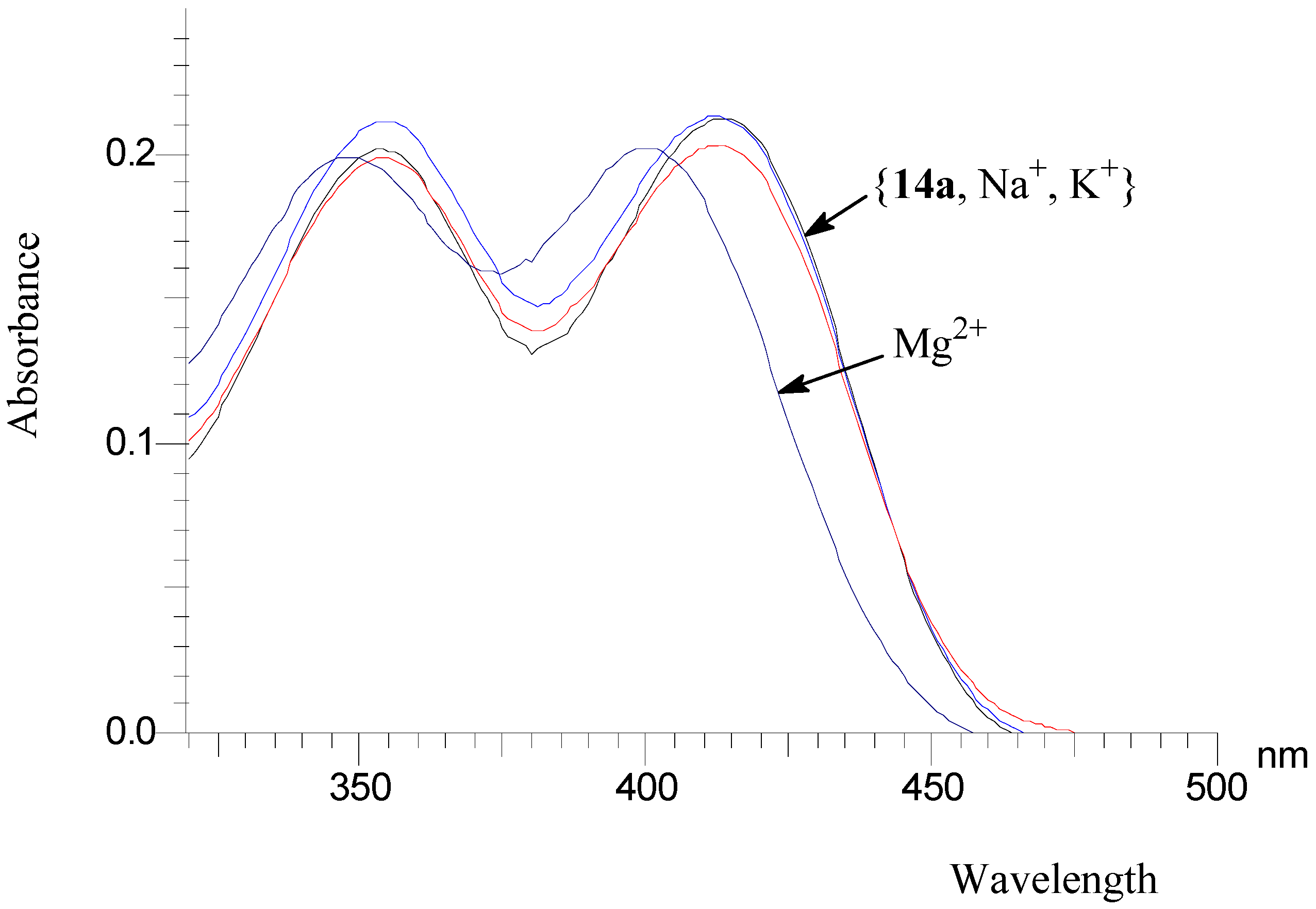
HIV integrase inhibitory activities of 4-oxo-4H-quinolizine-3-carboxylic acid derivatives
| Sample | Initial concentration (μg/mL) | IC50 (μg/mL) | Sample | Initial concentration (μg/mL) | IC50 (μg/mL) |
|---|---|---|---|---|---|
| S-y1 | 12 | 0.56 | 9a | 100 | -2 |
| 8a | 100 | -2 | 9b | 100 | -2 |
| 8b | 100 | -2 | 12 | 100 | -2 |
| 8c | 100 | -2 | 14a | 100 | -2 |
| 8d | 100 | -2 | 14b | 100 | -2 |
| 8e | 100 | -2 | 14c | 100 | -2 |
Conclusions
Experimental
General
General procedure for the synthesis of ethyl 1-(substituted phenylsulfonamido)-4-oxo-4H-quinolizine-3-carboxylates 6
General procedure for the synthesis of 1-(substituted phenylsulfonamido)-4-oxo-4H-quinolizine-3-carboxylic acids 8
General procedure for the synthesis of 7a,b
General procedure for the synthesis of 9a,b
Synthesis of ethyl 1-benzothiazol-2-yl-4-oxo-4H-quinolizine-3-carboxylate (11)
Synthesis of 1-benzothiazol-2-yl-4-oxo-4H-quinolizine-3-carboxylic acid (12)
General procedure for the synthesis of compounds 13a-c
General procedure for the synthesis of compounds 14a-c
Acknowledgements
References
- Turner, B.G.; Summers, M.F. Structural biology of HIV. J. Mol. Biol. 1999, 285, 1–32. [Google Scholar] [CrossRef]
- Molla, A.; Granneman, G.R.; Sun, E.; Kempf, D.J. Recent developments in HIV protease inhibitor therapy. Antiviral Res. 1998, 39, 1–23. [Google Scholar]
- Moyle, G.; Gazzard, B. Current knowledge and future prospects for the use of HIV protease inhibitors. Drugs 1996, 51, 701–702. [Google Scholar] [CrossRef]
- Pomerantz, R.J.; Horn, D.L. Twenty years of therapy for HIV 1 infection. Nature Med. 2003, 9, 867–873. [Google Scholar] [CrossRef]
- De Clercq, E. New developments in anti-HIV chemotherapy. Biochim. Biophys. Acta 2002, 1587, 258–275. [Google Scholar] [CrossRef]
- Brown, P.O. Integration of retroviral DNA. Curr. Top. Microbiol. Immunol. 1990, 157, 19–48. [Google Scholar]
- Katz, R.A.; Skalka, A.M. The retroviral enzymes. Annu. Rev. Biochem. 1994, 63, 133–173. [Google Scholar] [CrossRef]
- Farnet, C.M.; Wang, B.; lipford, J.R.; Bushman, F.D. Differential inhibition of HIV-1 preintegration complexes and purified integrase protein by small molecules. Proc. Natl. Acad. Sci. USA 1996, 93, 9742–9747. [Google Scholar] [CrossRef]
- Molteni, V.; Rhodes, D.; Rubins, K.; Hansen, M.; Bushman, F.D.; Siegel, J.S. A new class of HIV-1 integrase inhibitors: the 3,3,3',3'-tetramethyl-1,1'-spirobi(indan)-5,5',6,6'-tetrol family. J. Med. Chem. 2000, 43, 2031–2039. [Google Scholar] [CrossRef]
- Hazuda, D.J.; Felock, P.; Witmer, M.; Wolfe, A.; Stillmock, K.; Grobler, J.A.; Espeseth, A.; Gabryelski, L.; Schleif, W.; Blau, C.; Miller, M.D. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000, 287, 646–650. [Google Scholar] [CrossRef]
- Hazuda, D.J.; Yong, S.D.; Guare, J.P.; Anthony, N.J.; Gomez, R.P.; Wai, J.S.; Vacca, J.P.; Handt, L.; Motzel, S.L.; Klein, H.J.; Tussey, L.; Schleif, W.A.; Gabryelski, L.S.; Jin, L.; Miller, M.D.; Casimiro, D.R.; Emini, E.A.; Shiver, J.W. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science 2004, 305, 528–532. [Google Scholar] [CrossRef]
- Espeseth, A.S.; Felock, P.; Wolfe, A.; Witmer, M.; Grobler, J.; Anthony, N.; Egbertson, M.; Melamed, J.Y.; Young, S.; Hamill, T.; Cole, J.L.; Hazuda, D.J. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc. Natl. Acad. Sci. USA 2000, 97, 11244–11249. [Google Scholar] [CrossRef]
- Goldgur, Y.; Craigie, R.; Cohen, G.H.; Fujiwara, T.; Yoshinaga, T.; Fujishita, T.; Sugimoto, H.; Endo, T.; Murai, H.; Davies, D.R. Structure of the HIV-1 integrase catalytic domain complexed with an inhibitor: a platform for antiviral drug design. Proc. Natl. Acad. Sci. USA 1999, 96, 13040–13043. [Google Scholar] [CrossRef]
- Grobler, J.A.; Stillmock, K.; Hu, B.; Witmer, M.; Felock, P.; Espeseth, A.; Wolfe, A.; Egbertson, M.S.; Bourgeois, M.; Melamed, J.Y.; Wai, J.S.; Young, S.D.; Vacca, J.P.; Hazuda, D.J. Diketo acid inhibitor mechanism and HIV-1 integrase: implications for metal binding in the active site of phosphotransferase enzymes. Proc. Natl. Acad. Sci. USA 2002, 99, 6661–6666. [Google Scholar] [CrossRef]
- Zhuang, L.H.; Wai, J.S.; Embrey, M.W.; Fisher, T.E.; Egbertson, M.S.; Payne, L.S.; Juare, J.P., Jr.; Vacca, J.P.; Hazuda, D.J.; Felock, P.J.; Wolfe, A.L.; Stillmock, K.A.; Witmer, M.V.; Moyer, G.; Schleif, W.A.; Gabryelsk, L.J.; Leonard, Y.M.; Lynch, J.J., Jr.; Michelson, S.R.; Young, S.D. Design and Synthesis of 8-Hydroxy-[1,6]Naphthyridines as Novel Inhibitors of HIV-1 Integrase in Vitro and in Infected Cells. J. Med. Chem. 2003, 46, 453–456. [Google Scholar] [CrossRef]
- Summa, V.; Petrocchi, A.; Matassa, V.G.; Taliani, M.; Laufer, R.; Francesco, R.D.; Altamnra, S.; Pace, P. HCV NS5b RNA-Dependent RNA Polymerase Inhibitors: From α ,γ -Diketoacids to 4,5-Dihydroxypyrimidine- or 3-Methyl-5- hydroxypyrimidinonecarboxylic Acids. Design and Synthesis. J. Med. Chem. 2004, 47, 5336–5339. [Google Scholar] [CrossRef]
- Li, X.-M.; Zeng, C.-C.; Jiao, Z.-G.; Yan, H.; Zhong, R.-G. Synthesis and crystal structure of 7-acetamido-5-chloro-8-(p-methyloxybenzenesulfonyloxy)-2-styrylquinoline. Chinese J. Struct. Chem. 2007, 26, 669–673. [Google Scholar]
- Xu, Y.-S.; Zeng, C.-C.; Li, X.-M.; Zhong, R.-G.; Zeng, Y. Design, synthesis and Cu2+ recognition of β -diketoacid and quinoxalone derivatives bearing caffeoyl or galloyl moieties linked by arylamide as potential HIV integrase inhibitors. Chin. J. Chem. 2006, 24, 1086–1094. [Google Scholar] [CrossRef]
- Zeng, C.-C.; Li, X.-M.; Yan, H.; Zhong, R.-G. Design and synthesis of p/m-[p-(un)substituted phenylsulfonamido]phenyl β -diketo acids and quinoxalone derivatives. Chin. J. Chem. 2007, 25, 1174–1182. [Google Scholar] [CrossRef]
- Zeng, C.-C.; Liu, C.-F.; Zeng, J.; Zhong, R.-G. Electrochemical synthesis of 6-arylsulfonyl caffeic acid derivatives in aqueous medium. J. Electroanal. Chem. 2007, 608, 85–90. [Google Scholar] [CrossRef]
- Zeng, C.-C; Ping, D.-W.; Zhang, S.-C.; Zhong, R.-G.; Becker, J.Y. Electrochemical synthesis of polyhydroxylated aromatic derivatives substituted with mono- and dipyrimidinyl thioethers in aqueous medium. J. Electroanal. Chem. 2008, 622, 90–96. [Google Scholar] [CrossRef]
- Zeng, J.; Lv, X.-H.; Zeng, C.-C.; Hu, L.-M.; Zhong, R.-G. Design, synthesis and anti-HIV integrase evaluation of 1,2,3-triazol -4-yl-substituted 1,4-dihydro-4-oxo-1,5-napthyridine-3-carboxylic acids. Chin. J. Chem. 2009. (In press) [Google Scholar]
- Komatsu, H.; Iwasawa, N.; Citterio, D.; Suzuki, Y.; Kubota, T.; Tokuno, K.; Kitamura, Y.; Oka, K.; Suzuki, K. Design and synthesis of highly sensitive and selective fluorescein-derived magnesium fluorescent probes and application to intracellular 3D Mg2+ imaging. J. Am. Chem. Soc. 2004, 126, 16353–16360. [Google Scholar] [CrossRef]
- Otten, P.A.; London, R.E.; Levy, L.A. 4-Oxo-4H-quinolizine-3-carboxylic acids as Mg2+ selective, fluorescent indicators. Bioconjugate Chem. 2001, 12, 203–212. [Google Scholar] [CrossRef]
- Li, Q.; Chu, D.T.W.; Claiborne, A.; Cooper, C.S.; Lee, C.M.; Raye, K.; Berst, K.B.; Donner, P.; Wang, W.; Hasvold, L.; Fung, A.; Ma, Z.; Tufano, M.; Flamma, R.; Shen, L.L.; Baranowski, J.; Nilius, A.; Alder, J.; Meulbroek, J.; Marsh, K.; Crowell, D.; Hui, Y.; Seif, L.; Melcher, L.M.; Henry, R.; Spanton, S.; Faghih, R.; Klein, L.L.; Ken Tanaka, S.; Plattner, J.J. Synthesis and Structure-Activity Relationships of 2-Pyridones: A Novel Series of Potent DNA Gyrase Inhibitors as Antibacterial Agents. J. Med. Chem. 1996, 39, 3070–3088. [Google Scholar] [CrossRef]
- Oya, S.; Masuda, N.; Kuroki, Y.; Inoue, T.; Okudo, M.; Iwata, T.; Kokubo, K.; Mizuno, H.; Hagiwara, M. Preparation of 4-oxo-4H-quinolizine-3-carboxylic acid derivatives as antibacterial agents. Jpn. Kokai Tokkyo Koho 2003. JP 2003261566 A 20030919. [Google Scholar]
- Fukumoto, R.; Niwano, Y.; Kusakabe, H.; Chu, C.; Kimura, H.; Nagasawa, K.; Yanagihara, S.; Hirosawa, C.; Ishiduka, S. Preparation of 4-oxoquinolizines as antimicrobial agents. PCT Int. Appl. 2003. WO 2003029253. [Google Scholar]
- Iwamoto, K.; Araki, K.; Fujishima, H.; Shinkai, S. Fluorogenic calix[4]arene. J. Chem. Soc. Perkin Trans. 1. 1992, 1885–1887. [Google Scholar]
- Kim, Y.H.; Youk, J.S.; Moon, S.Y.; Choe, J.I.; Chang, S.K. Hg2+-selective fluorogenic chemosensor derived from 8-Aminoquinoline. Chem. Lett. 2004, 33, 702–703. [Google Scholar] [CrossRef]
- Kim, S.J.; Kool, E.T. Sensing Metal Ions with DNA Building Blocks: Fluorescent Pyridobenzimidazole Nucleosides. J. Am. Chem. Soc. 2006, 128, 6164–6171. [Google Scholar] [CrossRef]
- Weatherhead-Kloster, R.A.; Selby, H.D.; Miller, W.B., III; Mash, E.A. Organic Crystal Engineering with 1,4-Piperazine-2,5-diones. 6. Studies of the Hydrogen-Bond Association of Cyclo[(2-methylamino-4,7-dimethoxyindan-2-carboxylicacid)(2-amino-4,7-dimethoxyindan-2-carboxylic acid)]. J. Org. Chem. 2005, 70, 8693–8702. [Google Scholar] [CrossRef]
- Kawasuji, T.; Fuji, M.; Yoshinaga, T.; Sato, A.; Fujiwara, T.; Kiyama, R. A platform for designing HIV integrase inhibitors. A two-metal binding model as a potential mechanism of HIV integrase inhibitors. Bio. & Med. Chem. 2006, 14, 8420–8429. [Google Scholar]
- Sample Availability: Samples of compounds 6-14 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Y.-S.; Zeng, C.-C.; Jiao, Z.-G.; Hu, L.-M.; Zhong, R.-g. Design, Synthesis and Anti-HIV Integrase Evaluation of 4-Oxo-4H-quinolizine-3-carboxylic Acid Derivatives. Molecules 2009, 14, 868-883. https://doi.org/10.3390/molecules14020868
Xu Y-S, Zeng C-C, Jiao Z-G, Hu L-M, Zhong R-g. Design, Synthesis and Anti-HIV Integrase Evaluation of 4-Oxo-4H-quinolizine-3-carboxylic Acid Derivatives. Molecules. 2009; 14(2):868-883. https://doi.org/10.3390/molecules14020868
Chicago/Turabian StyleXu, Yi-Sheng, Cheng-Chu Zeng, Zi-Guo Jiao, Li-Ming Hu, and Ru-gang Zhong. 2009. "Design, Synthesis and Anti-HIV Integrase Evaluation of 4-Oxo-4H-quinolizine-3-carboxylic Acid Derivatives" Molecules 14, no. 2: 868-883. https://doi.org/10.3390/molecules14020868
APA StyleXu, Y.-S., Zeng, C.-C., Jiao, Z.-G., Hu, L.-M., & Zhong, R.-g. (2009). Design, Synthesis and Anti-HIV Integrase Evaluation of 4-Oxo-4H-quinolizine-3-carboxylic Acid Derivatives. Molecules, 14(2), 868-883. https://doi.org/10.3390/molecules14020868