Results and Discussion
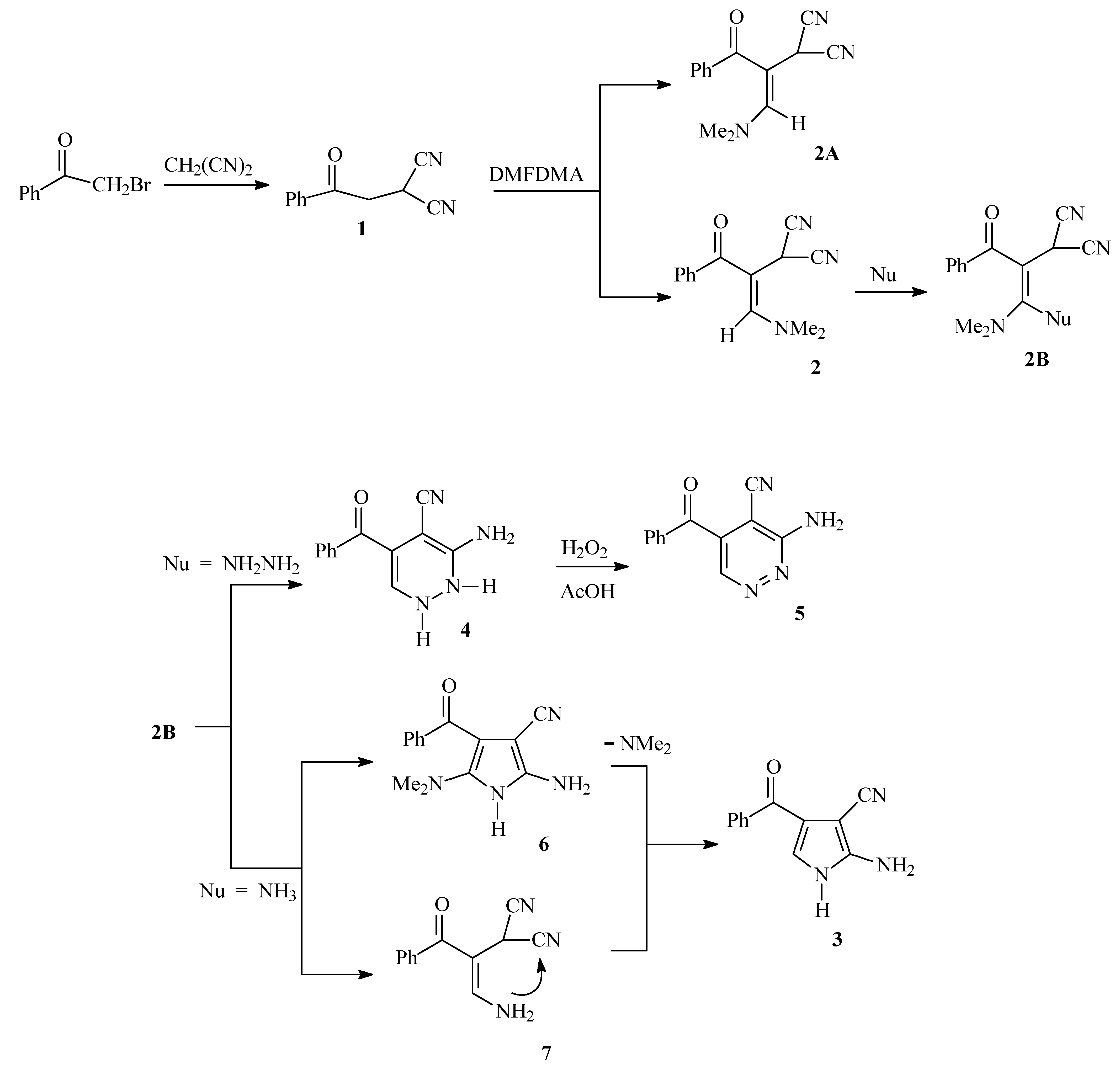
Phenacylmalononitrile has been prepared by treating malononitrile with phenacyl bromide. While the literature procedure [
12] afforded the desired product in 79 % yield, reaction of phenacyl bromide with malononitrile in ethanolic potassium hydroxide solution gave 2
-(2-oxo-2-phenylethyl) malononitrile (
1) in 85% yield. Heating phenacyl bromide and malononitrile in the presence of potassium hydroxide solution in a microwave oven at 85 °C gave an 80% yield of the target product. Reaction of
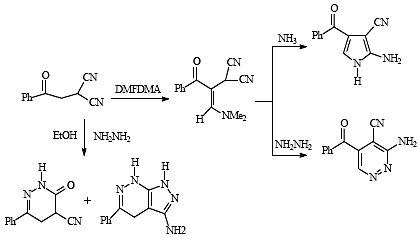
1 with dimethylformamide dimethyl acetal afforded 2-(3-(dimethylamino)-1-oxo-1-phenylprop-2-en-2-yl) malononitrile (
2) in 75% yield. Although
2 may also exist in the
Z form, only
2 (the
E form) was isolated according to the NMR NOE difference which indicated that the olefinic proton at δ = 8.44 ppm is not sterically proximal to the methylene proton at δ = 7.10 ppm. Refluxing
2 in acetic acid in presence of ammonium acetate afforded the aminopyrrole carbonitrile
3 in 60% yield. On the other hand, reaction of
2 with hydrazine hydrate afforded the aminopyridazine
4 that was readily oxidized to
5 upon treatment with H
2O
2 in acetic acid (
Scheme 1). It is assumed that initially ammonia adds across the double bond in
2 to yield an intermediate that then looses dimethylamine or alternatively, initially losses dimethylamine and then cyclizes to form
4 (
Scheme 1).
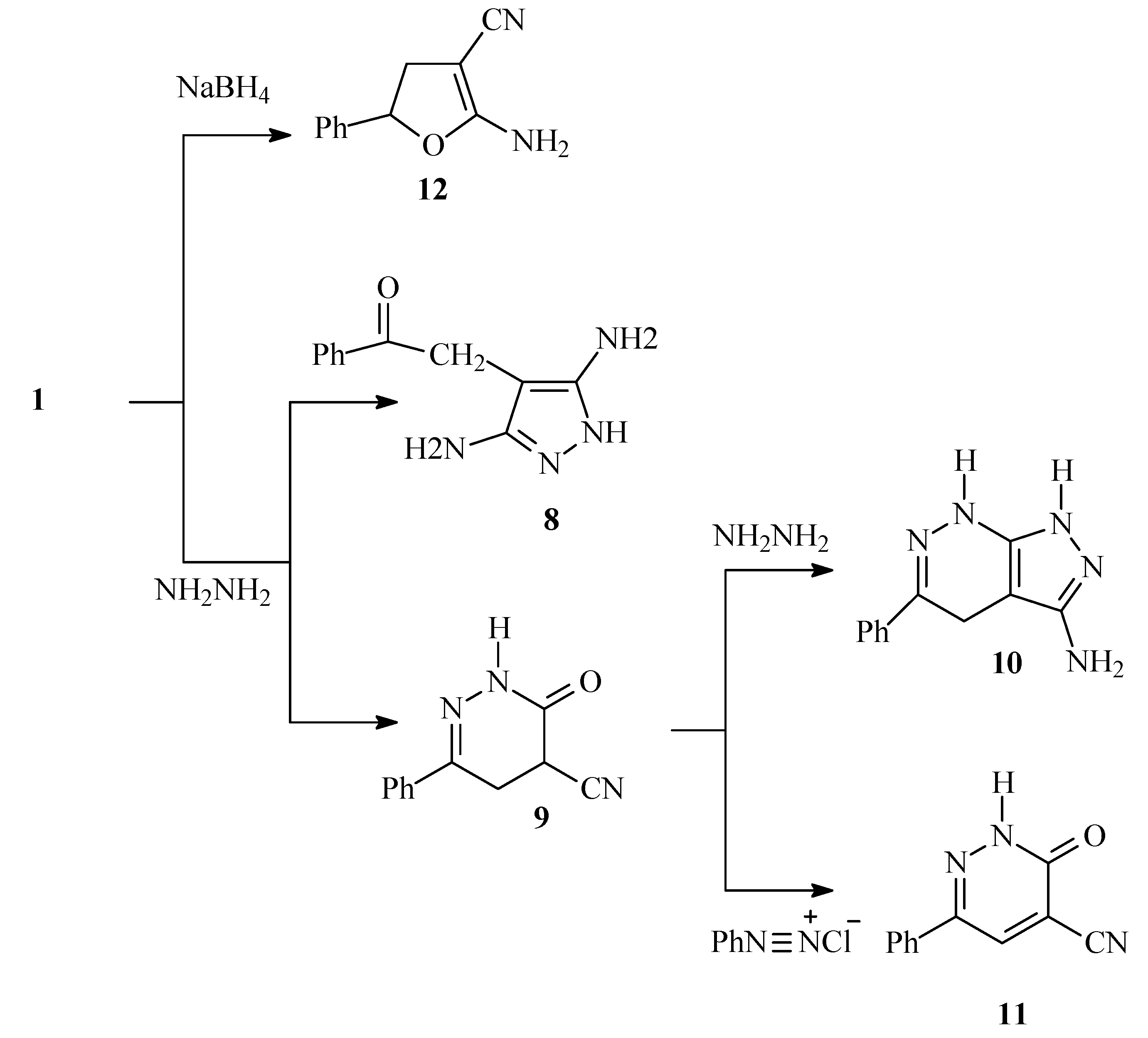
It has been previously reported by Abdelrazek
et al [
12] that (2-oxo-2-phenylethyl) malononitrile (
1) reacts with hydrazine hydrate to yield 4-phenacylpyrazole-3,5-diamine (
8). Subsequently Elnagdi
et al [
13] have shown that the major product of this reaction was in fact the 3-oxo-6-phenyl-2,3,4,5-tetrahydropyridazine-4-carbonitrile (
9). Very recently Abdelrazek [
14] claimed that in ethanolic solution a pyridazineimine was isolated. These should not be possible as water formed during the reaction would readily hydrolyze readily any imine possibly formed. However, they have also indicated that in refluxing ethanol other product was formed in less than 35% yield and assumed it to be
8, reported earlier by Abdelrazek
et al. [
12]
. Now we have found that in ethanol at room temperature
1 reacts with hydrazine hydrate to yield
9 as sole product in 96 % yield. When
9 was refluxed in ethanol with hydrazine hydrate a product of molecular formula C
11H
11N
5 (213.2) was formed. This proved identical with the product obtained by Abdelrazek [
12] or the one identified by Elnagdi
et al. [
13]. It thus became clear that
8 had never been isolated and that product believed earlier to be
8 really must have another structure. After inspection of the spectral and analytical data now wish to assign this product as 5-phenyl-4,7-dihydro-1
H-pyrazolo[3,4-c]pyridazin-3-ylamine (
10) obtained by further condensation of dihydropyridazine carbonitrile
9 with hydrazine hydrate (
Scheme 2). The NMR data of
10 suggest it is in a fast tautomeric equilibrium with the corresponding 2
H compound.
Scheme 1.
Syntheses of aminopyridazines and aminopyrrole carbonitrile.
Scheme 1.
Syntheses of aminopyridazines and aminopyrrole carbonitrile.
The dihydropyridazine
9 was readily oxidized to
11 on attempted coupling with benzenediazonium chloride. Compound
11 was also obtained upon oxidizing
9 in an AcOH-H
2O
2 mixture (
Scheme 2). Reduction of
1 with sodium borohydride in propanol solution afforded 2-amino-5-phenyl-4,5-dihydrofuran-3-carbonitrile (
12) in 60% yield (
Scheme 2). This compound has been synthesized earlier [
15] in almost the same way, but no spectral data had been reported.
1H-NMR and
13C-NMR of now reported for the first time.
Scheme 2.
Synthesis of 5-phenyl-4,7-dihydro-1H-pyrazolo[3,4-c]pyridazin-3-ylamine.
Scheme 2.
Synthesis of 5-phenyl-4,7-dihydro-1H-pyrazolo[3,4-c]pyridazin-3-ylamine.
We next shifted our interest to an exploration of the chemistry of 2-cyano-5-phenyl-3,5-dioxopentanonitrile, reported by Abdelrazek and Salah El-Din [
16] to be formed upon refluxing ethyl benzoyl acetate and malononitrile in ethanolic piperidine solution. Unfortunately, in our hands ethyl benzoylacetate did not react with malononitrile under the reported reaction conditions or even more vigorous ones. Thus it is concluded that 2-cyano-5-phenyl-3,5-dioxopentanonitrile could not have been obtained as reported. It is of interest that in the paper 2-cyano-5-phenyl-3,5-dioxopentanonitrile was claimed to be oil and the authors have not reported analytical data.
Experimental
General
All melting points are uncorrected and were determined on a Sanyo (Gallaenkamp) instrument. Infrared spectra were recorded in KBr on a Perkin-Elmer 2000 FT–IR system.1H-NMR and 13C-NMR spectra were determined on a Bruker DPX spectrometer operating at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR using in CDCl3 or DMSO as solvents and TMS as internal standard; chemical shifts are reported in δ (ppm). Mass spectra were measured on VG Autospec Q MS 30 and MS 9 (AEI) spectrometers, with EI 70 EV. Elemental analyses were measured by means of LEOCHNS-932 Elemental Analyzer. General purpose silica gel on polyester 20 x 20 cm TLC plates with UV indicator were used in TLC experiments.
Synthesis of 2-(2-oxo-2-phenylethyl)malononitrile (1)
A solution of malononitrile (0.66 g, 0.01 mol) and 2-bromo-1-phenylethanone (phenacyl bromide) (1.99 g, 0.01 mol) in ethanol (15 mL) was cooled to 0 oC. Potassium hydroxide (0.84 g, 0.15 mol) was added and the reaction mixture was stirred (followed until completion by TLC using 1:1 ethyl acetate- petroleum ether as eluent). The reaction was carefully quenched with ice-H2O and 1M HCl solution, The solid so formed was collected by filtration and recrystallized from ethanol to give a white product; yield 85 %; mp 160-62 °C; Anal. calcd. for C11H8N2O (184.2): C, 71.73; H, 4.38; N, 15.21. Found: C, 71.68; H, 4.35; N, 15.41; IR (KBr): υmax = 2211 (2CN), 1645 (CO); 1H-NMR (DMSO): δ, ppm = 4.11 (d, 2H, CH2, J = 8 Hz), 5.15 (t, 1H, CH, J = 8 Hz), 7.56-8.02 (m, 5H, Ar-H); 13C-NMR (DMSO): δ, ppm = 194.83, 135.29, 134.69, 129.41 (2C), 128.69 (2C), 114.88 (2CN), 38.75, 18.52. MS: m/z (%) 184 (M+, 50), 105 (100), 77 (70).
Synthesis of 2-(3-(dimethylamino)-1-oxo-1-phenylprop-2-en-2-yl)malononitrile (2)
A mixture of compound 1 (1.84 g, 0.01 mol) and N,N-dimethylformamide dimethyl acetal (DMFDMA, 1.19 g, 0.01 mol) in toluene (10 mL) was refluxed for 4 h. The reaction mixture was evaporated under reduced pressure yielding a crude product, which was recrystallized from toluene to give a light yellow product; yield 75 %; mp 129-30 °C; Anal. calcd. for C14H13N3O (239.2): C, 70.28; H, 5.48; N, 17.56. Found: C, 70.39; H, 5.50; N, 17.44; IR (KBr): υmax = 2208 (2CN), 1633 (CO); 1H-NMR (DMSO): δ, ppm = 3.05 (s, 3H, CH3), 3.14 (s, 3H, CH3), 7.10 (s, 1H, CH), 7.25-7.42 (m, 5H, Ar-H), 8.44 (s, 1H, CH); 13C-NMR (DMSO): δ, ppm = 194.47, 155.33, 134.89, 134.33, 129.43 (2C), 129.04 (2C), 106.93 (2CN), 78.17, 38.31, 37.44, 18.09; MS: m/z (%) 239 (M+, 100), 210 (15), 197 (45), 140 (25), 134 (90), 119 (20), 107 (15), 77 (15), 57 (25).
Synthesis of 2-amino-4-benzoyl-1H-pyrrole-3-carbonitrile (3)
A mixture of compound 2 (2.39 g, 0.01 mol) and ammonium acetate (1 g) in acetic acid (10 mL) was refluxed for 1 h. The mixture cooled and then was poured onto ice-water. The solid thus formed was collected by filtration and recrystallized from petroleum ether to give colorless crystals; yield 60 %; mp 297-98 °C. Anal. calcd. for C12H9N3O (211.2): C, 68.24; H, 4.29; N, 19.89. Found: C, 68.23; H, 4.31; N, 19.79; IR (KBr): υmax = 3350, 3330 (NH2), 3134 (NH), 2220 (CN), 1656 (CO); 1H-NMR (DMSO): δ, ppm = 7.33-7.80 (m, 8H, Ar-H, NH2, D2O exchangeable, NH, D2O exchangeable), 8.18 (s, 1H, CH); 13C-NMR (DMSO): δ, ppm = 189.57, 166.25, 158.65, 154.01, 150.03, 129.23 (2C), 128.76, 124.15 (2C), 102.14, 99.13; MS: m/z (%) 211 (M+, 100), 184 (80), 155 (80), 141 (25), 114 (10), 105 (25), 77 (45).
Synthesis of 3-amino-5-benzoyl-1,2-dihydropyridazine-4-carbonitrile (4)
A mixture of compound 2 (2.39 g, 0.01 mol) and hydrazine hydrate (0.5 g, 0.01 mol) in ethanol (10 mL) was refluxed for 5 h (monitored to completion by TIC using 1:1 ethyl acetate-petroleum ether as eluent). The mixture cooled and then was poured onto ice-water. The solid formed was collected by filtration and recrystallized from ethanol to give a yellow product; yield 65 %; mp 227-29 °C; Anal. calcd. for C12H10N4O (226.2): C, 63.71; H, 4.46; N, 24.76. Found: C, 63.55; H, 4.36; N, 24.89; IR (KBr): υmax = 3409, 3330 (NH2), 3080 (NH), 3060 (NH), 2200 (CN), 1608 (CO); 1H-NMR (DMSO): δ, ppm = 4.76 (br, 2H, NH2, D2O exchangeable), 7.39-7.82 (m, 8H, Ar-H), 8.20 (br, 1H, NH, D2O exchangeable), 9.09 (br, 1H, NH, D2O exchangeable); 13C-NMR (DMSO): δ, ppm = 192, 166.59, 160.54, 158.34, 149.83, 129.30 (2C), 129.18, 124.34 (2C), 109.24, 99.67; MS: m/z (%) 226 (M+, 100), 209 (10), 196 (25), 182 (20), 168 (10), 154 (25), 114 (10), 105 (20), 77 (20).
Synthesis of compound 3-amino-5-benzoylpyridazine-4-carbonitrile (5)
A mixture of 4 (2.26 g, 0.01 mol) and hydrogen peroxide 100% (3 mL) in acetic acid (10 mL) was refluxed for 2 h (followed to completion by TLC using 1:1 ethyl acetate-petroleum ether as eluent). The mixture was cooled and then poured onto ice-water. The solid formed was collected by filtration and recrystallized from ethanol to give a dark yellow product; yield 55 %; mp 148-150 °C; Anal. calcd. for C12H18N4O (226.2): C, 64.28; H,3.60; N, 24.99. Found: C, 64.02; H, 3.81; N, 24.75; IR (KBr): υmax = 3380, 3212 (NH2), 2281 (CN), 1643 (CO); 1H-NMR (DMSO): δ, ppm = 5.56 (br, 2H, NH2, D2O exchangeable), 7.57 -8.12 (m, 5H, Ar-H), 8.35 (s, 1H, CH); 13C-NMR (DMSO): δ, ppm = 191.32, 176.53, 154.54, 143.39, 129.99 (2C), 126.34 (2C), 121.54, 118.46, 111.42, 100.98; MS: m/z (%) 224 (M+, 75), 206 (15), 196 (50), 180 (25), 164 (10), 154 (35), 128 (10), 105 (45), 77 (50).
Syntheses of 3-oxo-6-phenyl-2,3,4,5-tetrahydropyridazine-4-carbonitrile (9) and 5-phenyl-4,7-dihydro-1H-pyrazolo[3,4-c]pyridazin-3-ylamine (10).
Procedure 1: A mixture of compound 1 (1.84 g, 0.01 mol) and hydrazine hydrate (0.50 g, 0.01 mol) in ethanol (10 mL) was stirred for 8 h at room temperature (followed by TLC until completion using ethyl acetate-petroleum ether 1:1 as eluent). The reaction mixture was poured onto ice-water. The solid product formed was collected by filtration and crystallized from ethanol to give white product 9 in 90% yield.
Procedure 2: A mixture of compound 1 (1.84 g, 0.01 mol) and hydrazine hydrate (0.50 g, 0.01 mol) in ethanol (10 mL) was refluxed for 5 h (followed by TLC until completion using ethyl acetate-petroleum ether 1:1 as eluent). The reaction mixture was cooled and poured onto ice-water. The solid product thus formed was collected by filtration and washed with hot ethanol to extract the white product 9. The residue was crystallized from N,N-dimethylformamide (DMF) to yield purple crystals of 10.
Compound 9: Yield 35 %; mp 253-55 °C. Anal. calcd. for C11H9N3O (199.2): C, 66.32; H, 4.55; N, 21.09. Found: C, 66.54; H, 4.32; N, 21.30. IR (KBr): υmax = 3234 (NH), 2154 (CN), 1693 (CO); 1H-NMR (DMSO): δ, ppm = 3.27 (dd, 1H, J = 12.6, 6.0), 3.59 (dd, 1H, J = 12.6, 6.0), 4.50 (dd, 1H, J = 13.2, 6.0), 7.45-7.79 (m, 5H, Ar-H), 11.49 (br, 1H, NH, D2O exchangeable); 13C-NMR (DMSO): δ, ppm = 159.81, 148.66, 134.91, 129.91, 126.99 (2C), 125.75 (2C), 116.92 (CN), 29.90, 25.61; MS: m/z (%) 199 (M+, 100), 170 (15), 155 (15), 140 (15), 115 (25), 103 (80), 77 (35).
Compound 10: Yield 65 %; mp 288-90 °C. Anal. calcd. for C11H11N5 (213.2): C, 61.96; H, 5.20; N, 32.84. Found: C, 61.77; H, 5.32; N, 32.65. IR (KBr): υmax = 3159, 3012 (NH2), 3012 (NH), 2983 (NH); 1H-NMR (DMSO): δ, ppm = 3.57 (s, 2H, 4-H), 7.33 (m, 1H, p-H, phenyl), 7.39 (m, 2H, m-H, phenyl), 7.70 (m, o-H, phenyl), 10.11 (br. s, 1H, 7-H, D2O exchangeable), the other NH signals are too broad to be localized; 13C-NMR (DMSO): δ, ppm = 154.1, 146.7 (C-5), 138.0 (br., C-3a and C-7a), 137.8 (i-C), 128.3 (m-C), 128.1 (p-C), 124.7 (o-C), 77.7 (C-3a), 21.3 (C-4); MS: m/z (%) 214 (M+, 100), 185 (35), 171 (15), 141 (15), 115 (25), 77 (35).
Synthesis of 3-Oxo-6-phenyl-2,3-dihydropyridazine-4-carbonitrile (11)
A cold solution of phenyldiazonium chloride (0.01 mol) was prepared by adding a solution of sodium nitrite (0.7 g into 10 mL H2O) to a cold solution of aniline hydrochloride (0.93 g, 0.01 mol of aniline in 5 mL concentrated HC1) with stirring at room temperature. The resulting solution of phenyldiazonium chloride was then added to a cold solution of compound 9 (1.99 g, 0.01 mol) in ethanol (50 mL) containing sodium acetate (2 g). The reaction mixture was stirred for 1 hr. The solid product formed was collected by filtration and crystallized from ethanol to give a yellow product, yield 70 %; mp 295-97 °C. Anal. calcd. for C11H7N3O (197.2): C, 67.00; H, 3.58; N, 21.31. Found: C, 66.98; H, 3.92; N, 21.65. IR (KBr): υmax = 3217 (NH), 2233 (CN), 1664 (CO); 1H-NMR (DMSO): δ, ppm = 7.46-7.91 (m, 5H, Ar-H), 8.85 (s, 1H, CH), 14.04 (br, 1H, NH, D2O exchangeable); 13C-NMR (DMSO): δ, ppm = 158.78, 148.35, 142.99, 138.34, 135.90, 130.34 (2C), 128.22 (2C), 119.26, 114.66. MS: m/z (%) 197 (M+, 100), 169 (65), 140 (95), 114 (35), 102 (40), 77 (35), 63 (20).
Synthesis of 2-amino-5-phenyl-4,5-dihydrofuran-3-carbonitrile (12)
A solution of compound 1 (1.84 g, 0.01 mol) in isopropanol (5 mL) was cooled to 0 oC. Sodium borohydride (0.756 g, 0.02 mol) was added and the reaction mixture was stirred for several hours (followed by TLC using ethyl acetate-petroleum ether 1:1 as eluent). If any starting compound was present, more sodium borohydride (up to 0.6 equivalents) was added. The reaction was carefully quenched with ice-H2O and 1M HCl solution, extracted with dichloromethane, filtered, and concentrated. The crude product was recrystallized from petroleum ether bp 60-80°C to give colorless crystals; yield 60 %; mp 116-18 °C. Anal. calcd. for C11H10N2O (186.21): C, 70.95; H, 5.41; N, 15.04. Found: C, 71.05; H, 5.36; N, 14.84. IR (KBr): υmax = 3321, 3267 (NH2), 2187 (CN); 1H-NMR (CDCl3): δ, ppm = 2.93 (dd, 1H, J = 12.4, 8), 3.313 (dd, 1H, J = 12.0, 8.0), 4.84 (br, 2H, NH2, D2O exchangeable), 5.64 (dd, 1H, J = 12.4, 8.0), 7.28-7.44 (m, 5H, Ar-H); 13C-NMR (DMSO): δ, ppm = 167.67, 140.60, 128.57 (2C), 128.23, 125.66 (2C), 119.98, 82.28, 46.39, 36.60. MS: m/z (%) 186 (M+, 100), 169 (45), 143 (80), 115 (95), 106 (15), 77 (45), 63 (15).