Substituted N-Phenylpyrazine-2-carboxamides: Synthesis and Antimycobacterial Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of antimycobacterial screening
3. Experimental Section
3.1. Chemistry
3.1.1. Instrumentation and chemicals
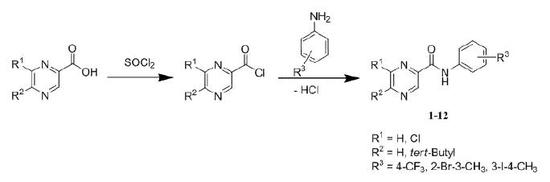
3.1.2. General procedure for the synthesis of compounds 1-12
3.2. Lipophilicity calculations
3.3. Biological methods
4. Conclusions
Acknowledgements
References and Notes
- Agrawal, Y.K.; Bhatt, H.G.; Raval, H.G.; Oza, P.M.; Vaidya, H.B.; Manna, K.; Gogoi, P. Emerging trends in tuberculosis therapy—A review. J. Sci. Indust. Res. 2007, 66, 191–208. [Google Scholar]
- Laughon, B.E. New tuberculosis drugs in development. Curr. Topics Med. Chem. 2007, 7, 463–473. [Google Scholar] [CrossRef]
- Doležal, M. Biologically active pyrazines of natural and synthetic origin. Chem. Listy 2006, 100, 959–966. [Google Scholar]
- Speirs, R.J.; Welch, J.T.; Cynamon, M.H. Activity of n-propyl pyrazinoate against pyrazinamide-resistant mycobacterium-tuberculosis: Investigation into mechanism of action and mechanism of resistence to pyrazinamide. Antimicrob. Agents Chemother. 1995, 39, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wade, M.M.; Scorpio, A.; Zhang, H.; Sun, Z.H. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 2003, 52, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.C.; Zimhony, O.; Chung, W.J.; Sayahi, H.; Jacobs, W.R.; Welch, J.T. Inhibition of isolated mycobacterium tuberculosis fatty acid synthase I by pyrazinamide analogs. Antimicrob. Agents Chemother. 2007, 51, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Doležal, M.; Hartl, J.; Miletín, M.; Macháček, M.; Kráľová, K. Synthesis and photosynthesis-inhibiting activity of some anilides of substituted pyrazine-2-carboxylic acids. Chem. Pap. 1999, 53, 126–128. [Google Scholar] [CrossRef]
- Doležal, M.; Vičík, R.; Miletín, M.; Kráľová, K. Synthesis and antimycobacterial, antifungal, and photosynthesis-inhibiting evaluation of some anilides of substituted pyrazine-2-carboxylic acids. Chem. Pap. 2000, 54, 245–248. [Google Scholar] [CrossRef]
- Doležal, M.; Tůmová, L.; Kešetovičová, D.; Tůma, J.; Kráľová, K. Substituted N-phenylpyrazine-2-carboxamides, their synthesis and evaluation as herbicides and abiotic elicitors. Molecules 2007, 12, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Doležal, M.; Palek, L.; Vinšová, J.; Buchta, V.; Jampílek, J.; Kráľová, K. Substituted pyrazinecarboxamides; synthesis and their biological evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Doležal, M.; Čmedlová, P.; Palek, L.; Vinšová, J.; Kuneš, J.; Buchta, V.; Jampílek, J.; Kráľová, K. Synthesis and antimycobacterial evaluation of substituted pyrazinecarboxamides. Eur. J. Med. Chem. 2008, 43, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Ancizu, S.; Moreno, E.; Torres, E.; Burguete, A.; Pérez-Silanes, S.; Benítez, D.; Villar, R.; Solano, B.; Marín, A.; Aldana, I.; Cerecetto, H.; González, M.; Monge, A. Heterocyclic-2-carboxylic acid (3-Cyano-1,4-di-N-oxidequinoxalin-2-yl)amide derivatives as hits for the development of neglected disease drugs. Molecules 2009, 14, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Cynamon, M.H.; Speirs, R.J.; Welch, J.T. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob Agents Chemother. 1998, 42, 462–463. [Google Scholar] [PubMed]
- Wade, M.M.; Zhang, Y. Effects of weak acids, UV and proton motive force inhibitors on pyrazinamide activity against Mycobacterium tuberculosis in vitro. J. Antimicrob. Chemother. 2006, 58, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Shigeta, Y.; Uchimaru, F.; Okada, S.; Ozasayama, E. Methyl 6-methoxypyrazine-2-carboxylate. JP Pat. 44012898 1969. [Chem. Abstr. 1969, 71, 112979y.]. [Google Scholar]
- Doležal, M.; Hartl, J.; Lyčka, A.; Buchta, V.; Odlerová, Ž. Synthesis and antituberculotic properties of some substituted pyrazinecarbothioamides. Collect. Czech. Chem. Commun. 1996, 61, 1102–1108. [Google Scholar] [CrossRef]
- TAACF. 2009. Available online: http://www.taacf.org/Process-text.htm#nhdp-text accessed 27 August, 2009.
Sample Availability: Samples of the compounds 1-12 are available from the authors. |



| Compd. | Regional Hospital in Pardubice (Czech Republic) | TAACF (USA) | ||||
|---|---|---|---|---|---|---|
| MIC [mg/L-1] | M. tuberculosis H37Rv | |||||
| M. tuberculosis H37Rv | M. kansasii CNCTC My 235/80 | M. avium CNCTC My 66/72 | M. avium 152/74 | IC50 [μg/mL] | IC90 [μg/mL] | |
| 1 | 2 | 128 | >128 | >128 | >100 | >100 |
| 2 | 8 | 32 | >128 | >128 | >100 | >100 |
| 3 | 8 | >128 | >128 | >128 | >100 | >100 |
| 4 | 8 | >128 | >128 | >128 | >100 | 20.703 |
| 5 | 2 | >128 | >128 | >128 | >100 | >100 |
| 6 | >128 | >128 | >128 | >128 | >100 | >100 |
| 7 | 32 | >128 | >128 | >128 | >100 | >100 |
| 8 | 16 | >128 | >128 | >128 | >100 | >100 |
| 9 | <2 | >128 | >128 | >128 | >100 | 69.099 |
| 10 | 8 | >128 | >128 | >128 | >100 | >100 |
| 11 | 8 | >128 | >128 | >128 | >100 | >100 |
| 12 | 4 | >128 | >128 | >128 | 0.728 | 0.819 |
| PZA | 8 | >128 | >128 | >128 | -a | >20b |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doležal, M.; Zitko, J.; Kešetovičová, D.; Kuneš, J.; Svobodová, M. Substituted N-Phenylpyrazine-2-carboxamides: Synthesis and Antimycobacterial Evaluation. Molecules 2009, 14, 4180-4189. https://doi.org/10.3390/molecules14104180
Doležal M, Zitko J, Kešetovičová D, Kuneš J, Svobodová M. Substituted N-Phenylpyrazine-2-carboxamides: Synthesis and Antimycobacterial Evaluation. Molecules. 2009; 14(10):4180-4189. https://doi.org/10.3390/molecules14104180
Chicago/Turabian StyleDoležal, Martin, Jan Zitko, Diana Kešetovičová, Jiří Kuneš, and Michaela Svobodová. 2009. "Substituted N-Phenylpyrazine-2-carboxamides: Synthesis and Antimycobacterial Evaluation" Molecules 14, no. 10: 4180-4189. https://doi.org/10.3390/molecules14104180
APA StyleDoležal, M., Zitko, J., Kešetovičová, D., Kuneš, J., & Svobodová, M. (2009). Substituted N-Phenylpyrazine-2-carboxamides: Synthesis and Antimycobacterial Evaluation. Molecules, 14(10), 4180-4189. https://doi.org/10.3390/molecules14104180