A New Atisane-Type Diterpene from the Bark of the Mangrove Plant Excoecaria Agallocha
Abstract
:Introduction
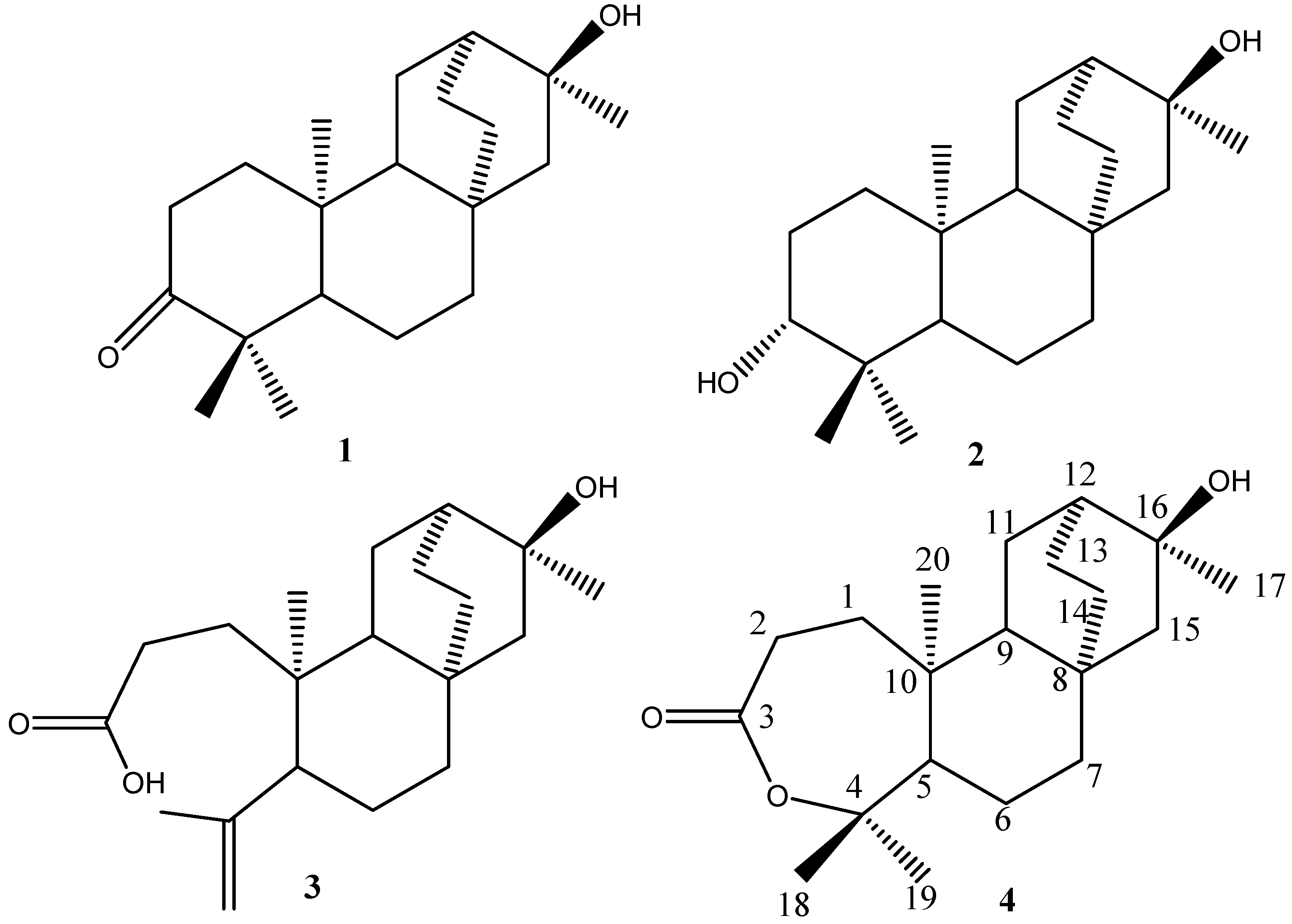
Results and Discussion
 : -40°(c=1.0, MeOH). The molecular formula of C20H32O3 was established by HR-ESI-MS at m/z 343.22502 ([M+Na] +). The ESI-MS of 4 showed quasi-molecular ion peaks at m/z 343 ([M+Na] +). Analysis of the 1H-NMR, 13C- NMR, DEPT and HMQC spectral data (Table 1) revealed the presence of four methyls (δc 30.0, 32.7, 24.6, 14.4), eight methylenes, three methines and five quaternary carbons (including a carbonyl group at δc 175.1 and two oxygenated carbons at δc 86.0, 71.3). The NMR spectra of 4 were similar to those of 2, except for the fact that the peaks of C-3 (δC 175.1, s) and C-4 (δC 86.0, s) were shifted upfield, compared with those of 2 (δC-3 38.7, s and δC-4 79.1, s), indicating that the oxygenated methine (C-3) and the quaternary carbon (C-4) of 2 were replaced by a carbonyl group (C-3) and an oxygenated quaternary carbon (C-4). The peak of C-4 (δC86.0, s) was shifted upfield compared with the carbon bearing OH group at δC 79.1 but was similar to the carbon bearing a lactone group at δC 84.8 [24]. This revealed the lactone between C-3 and C-4. Furthermore, H-2 showed long-correlations with a quaternary carbon at δ 175.1 (C-3), a methylene at δ 35.3 (C-1), and a quaternary carbon at δ 40.0 (C-10), H-12 showed long-range correlations with δ 49.7 (C-9), δ 26.3 (C-14), and δ 30.0 (C-17), H-14 showed long-range correlations with δ 49.7 (C-9), δ 56.6 (C-15), and δ 38.3 (C-7). In addition, other correlations for the quaternary carbons (C-4, C-8, C-10 and C-16) were also observed in the HMBC spectrum (Table 1). The H, H-COSY spectra revealed the connectivity between C-1/C-2, C-9/C-11 and C-13/C-14 (Figure 2, Table 1).
: -40°(c=1.0, MeOH). The molecular formula of C20H32O3 was established by HR-ESI-MS at m/z 343.22502 ([M+Na] +). The ESI-MS of 4 showed quasi-molecular ion peaks at m/z 343 ([M+Na] +). Analysis of the 1H-NMR, 13C- NMR, DEPT and HMQC spectral data (Table 1) revealed the presence of four methyls (δc 30.0, 32.7, 24.6, 14.4), eight methylenes, three methines and five quaternary carbons (including a carbonyl group at δc 175.1 and two oxygenated carbons at δc 86.0, 71.3). The NMR spectra of 4 were similar to those of 2, except for the fact that the peaks of C-3 (δC 175.1, s) and C-4 (δC 86.0, s) were shifted upfield, compared with those of 2 (δC-3 38.7, s and δC-4 79.1, s), indicating that the oxygenated methine (C-3) and the quaternary carbon (C-4) of 2 were replaced by a carbonyl group (C-3) and an oxygenated quaternary carbon (C-4). The peak of C-4 (δC86.0, s) was shifted upfield compared with the carbon bearing OH group at δC 79.1 but was similar to the carbon bearing a lactone group at δC 84.8 [24]. This revealed the lactone between C-3 and C-4. Furthermore, H-2 showed long-correlations with a quaternary carbon at δ 175.1 (C-3), a methylene at δ 35.3 (C-1), and a quaternary carbon at δ 40.0 (C-10), H-12 showed long-range correlations with δ 49.7 (C-9), δ 26.3 (C-14), and δ 30.0 (C-17), H-14 showed long-range correlations with δ 49.7 (C-9), δ 56.6 (C-15), and δ 38.3 (C-7). In addition, other correlations for the quaternary carbons (C-4, C-8, C-10 and C-16) were also observed in the HMBC spectrum (Table 1). The H, H-COSY spectra revealed the connectivity between C-1/C-2, C-9/C-11 and C-13/C-14 (Figure 2, Table 1).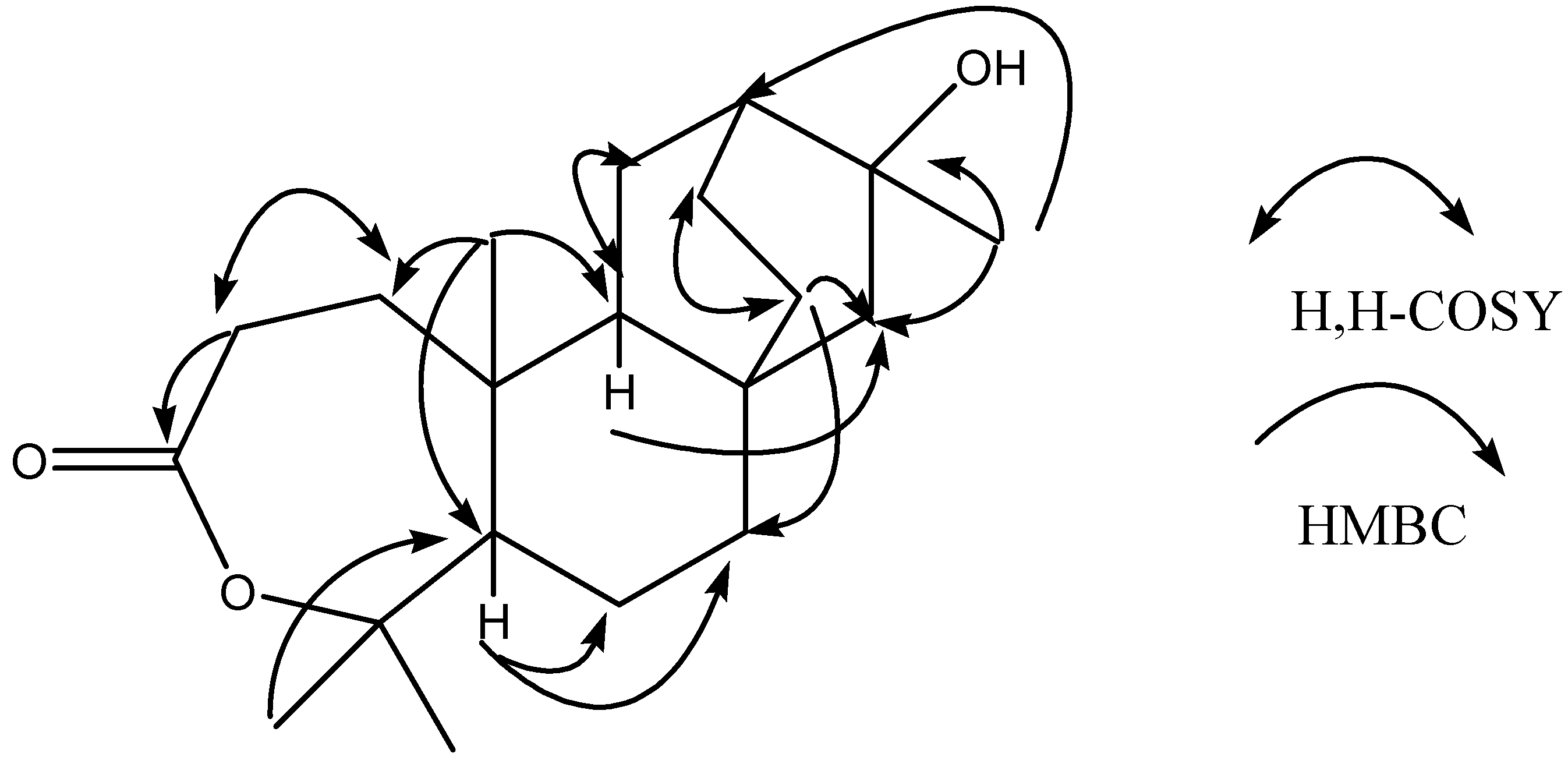
| No. | 13C | 1H | HMBC | H, H-COSY |
|---|---|---|---|---|
| 1 | 35.3 t | 1.72, m | 175.1, 55.7, 49.7, 40.0, 31.2, 14.4 | 2.64 |
| 1.47, m | 175.1, 49.7, 40.0, 31.2, 14.4 | 2.64 | ||
| 2 | 31.2 t | 2.64, m | 175.1, 40.0, 35.5 | 1.72, 1.47 |
| 3 | 175.1 s | |||
| 4 | 86.0 s | |||
| 5 | 55.7 d | 1.63, dd, (3.9, 10.4) | 86.0, 49.7, 40.0, 38.3, 35.3, 32.7, 24.6, 22.1, 14.4 | |
| 6 | 22.1 t | 1.49, m | 55.7, 40.0, 38.3, 33.2 | |
| 7 | 38.3 t | 1.39, m | 56.6, 49.7, 33.2, 26.3, 22.1 | |
| 1.20, m | 56.6, 49.7, 33.2, 26.3, 22.1 | |||
| 8 | 33.2 s | |||
| 9 | 49.7 d | 1.35, ddd, (1.5, 7.0, 11.4) | 56.6, 40.0, 35.3, 33.2, 26.3, 23.3, 14.4 | 2.09 |
| 10 | 40.0 s | |||
| 11 | 23.3 t | 2.09, m | 71.3, 49.7, 40.0, 37.5, 33.2, 23.2 | 1.35 |
| 1.18, m | 71.3, 37.5 | |||
| 12 | 37.5 d | 1.58, m | 71.3, 56.6, 49.7, 30.0, 26.3, 23.3 | |
| 13 | 23.2 t | 1.69, m | 71.3, 37.5, 33.2, 26.3, 23.3 | 1.84, 0.87 |
| 1.48, m | 71.3, 37.5, 33.2, 26.3, 23.3 | 1.84, 0.87 | ||
| 14 | 26.3 t | 1.84, m | 56.6, 37.5, 33.2, 23.2 | 1.69, 1.48 |
| 0.87, m | 56.6, 49.7, 33.2, 23.2 | 1.69, 1.48 | ||
| 15 | 56.6 t | 1.35, m | 71.3, 49.7. 38.3, 33.2, 30.0, 26.3 | |
| 1.24, m | 71.3, 49.7. 38.3, 33.2, 30.0, 26.3 | |||
| 16 | 71.3 s | |||
| 17 | 30.0 q | 1.31, s | 71.3, 56.6, 37.5 | |
| 18 | 32.7 q | 1.46, s | 86.0, 55.7, 24.6 | |
| 19 | 24.6 q | 1.45, s | 86.0, 55.7, 32.7 | |
| 20 | 14.4 q | 1.17, s | 55.7, 49.7, 40.0, 35.3 |


Experimental
General
Extraction and Isolation
Anti-microfouling Assay
Acknowledgements
References
- Lin, P. Mangrove vegetation; China Ocean Press: Beijng, China, 1988; pp. 51–52. [Google Scholar]
- Bandaranayake, W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Kawashima, T.; Takahashi, T.; Inoue, Y.; Kodama, M.; Ito, S. Euphorbiaceae: Constitiuents of Excoecaria agallocha. Phytochemistry 1971, 10, 3308–3309. [Google Scholar] [CrossRef]
- Ohigashi, H.; Katsumata, H.; Kawazu, K. A piscicidal constituent of Excoecaria agallocha. Agric. Biol. Chem. 1974, 38, 1393–1095. [Google Scholar] [CrossRef]
- Prakash, S.; Khan, M.A.; Khan, H.; Zamanm, A. A piperdine alkaloid from Excoecaria agallocha. Phytochemistry 1983, 22, 1836–1837. [Google Scholar] [CrossRef]
- Weislow, O.S.; Kiser, R.; Fine, D.L.; Bader, J.; Shoemaker, R.H.; Boyd, M.R. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 1989, 81, 577–586. [Google Scholar] [CrossRef]
- Konishi, T.; Takasaki, M.; Tokuda, H.; Kiyosawa, S.; Konoshima, T. Anti-tumor-promoting activity of diterpenes from Excoecaria agallocha. Biol. Pharm. Bull. 1998, 21, 993–996. [Google Scholar] [CrossRef]
- Konoshima, T.; Konishi, T.; Takasaki, M.; Yamazoe, K.; Tokuda, H. Anti-tumor-promoting activity of the diterpene from Excoecaria agallocha II. Biol. Pharm. Bull. 2001, 24, 1440–1442. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Chen, J.D.; Feng, D.Q.; Yang, Z.W.; Wang, Z.C.; Qiu, Y.; Lin, Y.M. Antifouling Metabolites from the Mangrove Plant Ceriops tagal. Molecules 2008, 13, 212–219. [Google Scholar]
- Gerhardt, D.J.; Rittschof, D.; Mayo, S.W. Chemical ecology and the search for antifoulants. J. Chem. Ecol. 1988, 14, 1903–1915. [Google Scholar]
- Costerton, J.W. The Biofilm Primer; Berlin, Heidelberg: Springer-Verlag, USA, 2007. [Google Scholar]
- McBain, A.J.; Allison, D.; Gilbert, P. Emerging strategies for the chemical treatment of microbial biofilms. Biotechnol. Genetic. Eng. Rev. 2000, 17, 267–279. [Google Scholar]
- Wahl, M.; Kröger, K.; Lenz, M. Non-toxic protection against epibiosis. Biofouling 1998, 12, 205–226. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Richmond, M.D.; Seed, R. A review of marine macrofouling communities with special reference to animal fouling. Biofouling 1991, 2, 151–168. [Google Scholar] [CrossRef]
- Lin, P.; Fu, Q. Environmental Ecology and Economic Utilization of Mangroves in China; China Higher Education Press: Beijing; Springer-Verlag Berlin: Heidelberg, 2000; pp. 1–3. [Google Scholar]
- Ross, P.M. Larval supply, settlement and survival of barnacles in a temperate mangrove forest. Mar. Ecol. Prog. Ser. 2001, 21, 237–249. [Google Scholar] [CrossRef]
- Kang, J.; Chen, R.Y.; Yu, D.Q. A new isopimarane-type diterpene and a new natural atisanetype diterpene from Excoecaria agallocha. J. Asian Nat. Prod. Res. 2005, 7, 729–734. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Vanderah, D.J.; Hollenbeak, K.H.; Enwall, C.E.L.; Gopichand, Y.; Sengupta, P.K.; Hossain, M.B.; Van Der Helm, D. Metabolites from the marine sponge Tedania ignis. a new atisanediol and several known diketopiperazines. J. Org. Chem. 1983, 48, 3941–3945. [Google Scholar] [CrossRef]
- Konishi, T.; Yamazoe, K.; Kanzato, M.; Konoshima, T.; Fujiwara, Y. Three diterpenoids (Excoecarins V1—V3) and a flavanone glycoside from thefresh stem of Excoecaria agallocha. Chem. Pharm. Bull. 2003, 51, 1142–1146. [Google Scholar] [CrossRef]
- Wang, J.D.; Li, Z.Y.; Guo, Y.W. Secoatisane- and isopimarane-type diterpenoids from the chinese mangrove Excoecaria agallocha L. Helv. Chim. Acta 2005, 88, 979–985. [Google Scholar] [CrossRef]
- Wang, J.D.; Li, Z.Y.; Xiang, W.S.; Guo, Y.W. Further New Secoatisane Diterpenoids from the Chinese Mangrove Excoecaria agallocha L. Helv. Chim. Acta 2006, 89, 1367–1372. [Google Scholar] [CrossRef]
- Lee, K.H.; Yang, M.C.; Kim, K.H.; Kwon, H.C.; Choi, S.U.; Lee, K.R. A New Phenolic Amide from the Roots of Paris verticillata. Molecules 2008, 13, 41–45. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Meyer, R.L.; Laursen, B.S.; Shipovskov, S.; Besenbacher, F.; Poulsen, C.H. Antifouling enzymes and the biochemistry of marine settlement. Biotechnol. Adv. 2008, 26, 471–481. [Google Scholar]
- Leroy, C.; Delbarre, C.; Ghillebaert, F.; Compere, C.; Combes, D. Effects of commercial enzymes on the adhesion of a marine biofilm-forming bacterium. Biofouling 2008, 24, 11–22. [Google Scholar] [CrossRef]
- Finney, D.J. The Spearman-Kaerber method. In Statistical Methods in Biological Assay; Charles Griffin & Co., Ltd.: London, UK, 1952; pp. 524–530. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.C.; Lin, Y.M.; Feng, D.Q.; Ke, C.H.; Lin, P.; Yan, C.L.; Chen, J.D. A New Atisane-Type Diterpene from the Bark of the Mangrove Plant Excoecaria Agallocha. Molecules 2009, 14, 414-422. https://doi.org/10.3390/molecules14010414
Wang ZC, Lin YM, Feng DQ, Ke CH, Lin P, Yan CL, Chen JD. A New Atisane-Type Diterpene from the Bark of the Mangrove Plant Excoecaria Agallocha. Molecules. 2009; 14(1):414-422. https://doi.org/10.3390/molecules14010414
Chicago/Turabian StyleWang, Zhan Chang, Yi Ming Lin, Dan Qin Feng, Cai Huan Ke, Peng Lin, Chong Ling Yan, and Jun De Chen. 2009. "A New Atisane-Type Diterpene from the Bark of the Mangrove Plant Excoecaria Agallocha" Molecules 14, no. 1: 414-422. https://doi.org/10.3390/molecules14010414




