Conversion of Oximes to Carbonyl Compounds by Triscetylpyridinium Tetrakis(oxodiperoxotungsto) Phosphate (PCWP)-mediated Oxidation with Hydrogen Peroxide
Abstract
:Introduction
Results and Discussion
| Oxime | R1 | R2 | Time (min.) | Conversion (%) | Yield (%)b,c |
|---|---|---|---|---|---|
| 1a | C6H5 | H | 80 | 90 | 100 |
| 1b | p-Cl-C6H4 | H | 70 | 95 | 100 |
| 1c | C6H5 | CH3 | 90 | 80 | 95 |
| 1d | CH(Me)(Et) | H | 60 | 95 | 70 |
| 1e | n-C7H15 | H | 60 | 95 | 70 |
| 1f | –(C5H10)– | 90 | 85 | 93 | |
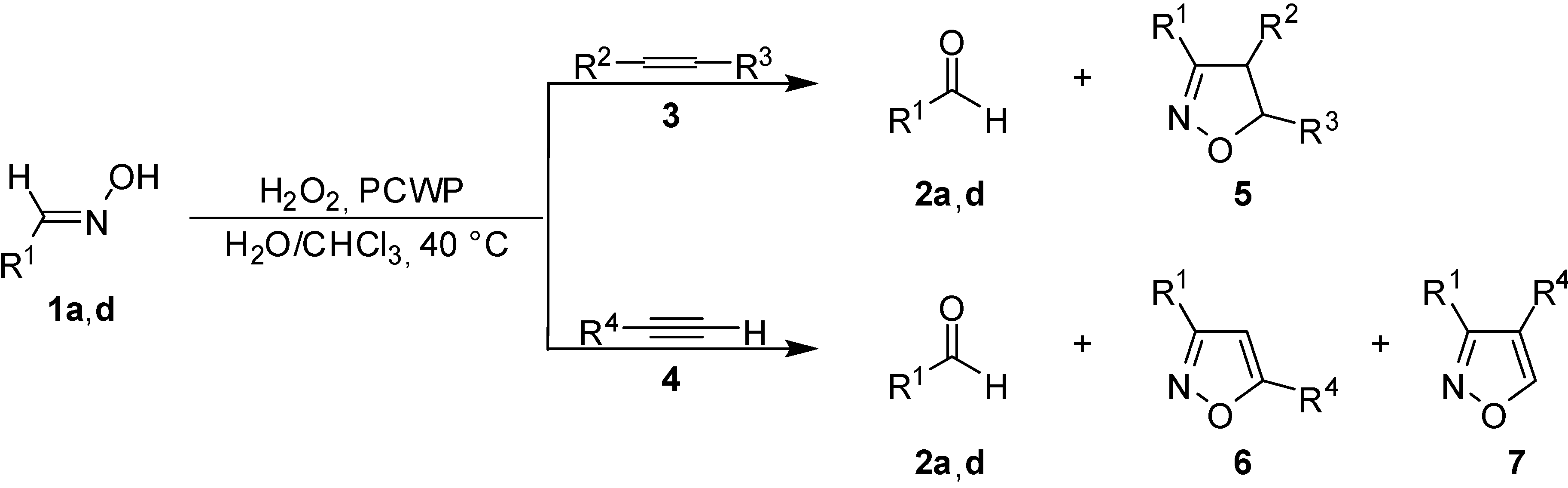
| Entry | R1 | R2 | R3 | R4 | Time (h) | Conv. (%) | Yields, (%)b,c | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 6 | 7 | |||||||
| 1 | C6H5 | H | CH3(CH2)5 | 9 | 84 | 62 | 17 | |||
| 2 | CH(Me)(Et) | H | CH3(CH2)5 | 5 | 89 | 56 | 6 | |||
| 3 | C6H5d | CO2Me | CO2Me | 9 | 81 | 43 | 46 | |||
| 4 | CH(Me)(Et)e | CO2Me | CO2Me | 6 | 100 | 30 | 30 | |||
| 5 | C6H5 | C6H5 | 16 | 71 | 67 | 28 | ||||
| 6 | CH(Me)(Et) | C6H5 | 6.5 | 90 | 49 | 9 | ||||
| 7 | C6H5 | CH3(CH2)5 | 6.5 | 83 | 49 | 32 | ||||
| 8 | CH(Me)(Et) | CH3(CH2)5 | 3 | 88 | 33 | 5 | ||||
| 9 | C6H5 | CO2Me | 8 | 86 | 30 | 42 | 13 | |||
| 10 | CH(Me)(Et) | CO2Me | 3 | 100 | 43 | 25 | 8 | |||

Conclusions
Experimental
General
Preparation of triscetylpyridinium tetrakis(diperoxotungsto)phosphate (PCWP)
Oxidation and cycloaddition reactions: general procedure for oxidation
General procedure for oxidation-cycloaddition
Acknowledgements
References and Notes
- Green, T.W.; Wuts, P.G. Protective Groups in Organic Synthesis, 3rd ed.; John Wiley and Sons: New York, 1991. [Google Scholar]
- Barton, D.H.R.; Beaton, J.M. A Synthesis of Aldosterone Acetate. J. Am. Chem. Soc. 1961, 83, 4083–4089. [Google Scholar] [CrossRef]
- Kadzyauskas, P. P.; Zefirov, N. S. Nitrosochlorination of Alkenes. Russ. Chem. Rev. (Engl. Transl.) 1968, 37, 543–550. [Google Scholar] [CrossRef]
- Mukai, C.; Hanaoka, M. Development of Highly Stereoselective and Regioselective Reactions Based on the Alkyne-Co2(CO)6 Complexes. Synlett 1996, 11–17. [Google Scholar] [CrossRef]
- Czekelius, C.; Carreira, E. M. Convenient Transformation of Optically Active Nitroalkanes into Chiral Aldoximes and Nitriles. Angew. Chem. Int. Ed. 2005, 44, 612–615. [Google Scholar] [CrossRef]
- Corsaro, A.; Chiacchio, U.; Pistarà, V. Regeneration of Carbonyl Compounds from the Corresponding Oximes. Synthesis 2001, 1903–1931. [Google Scholar] [CrossRef]
- Karami, B.; Montazerozohori, M. Tungstate Sulfuric Acid (TSA)/KMnO4 as a Novel Heterogeneous System for Rapid Deoximation. Molecules 2006, 11, 720–725. [Google Scholar] [CrossRef]
- Li, G.; Ding, Y.; Wang, J.; Wang, X.; Suo, J. New Progress of Keggin and Wells–Dawson Type Polyoxometalates Catalyze Acid and Oxidative Reactions. J. Mol. Catal. A 2007, 262, 67–76. [Google Scholar]
- Ballistreri, F. P.; Chiacchio, U.; Rescifina, A.; Tomaselli, G. A.; Toscano, R. M. One-Flask Transformation of Secondary Amines to Nitrones by Oxidation with Hydrogen Peroxide Mediated by Triscetylpyrydinium Tetrakis Oxodiperoxotungsto-Phosphate (PCWP): Some Mechanistic Considerations. Tetrahedron 1992, 48, 8677–8684. [Google Scholar] [CrossRef]
- Carraro, M.; Sandei, L.; Sartorel, A.; Scorrano, G.; Tonchio, M. Hybrid Polyoxotungstates as Second-Generation POM-Based Catalysts for Microwave-Assisted H2O2 Activation. Org. Lett. 2006, 8, 3671–3674. [Google Scholar] [CrossRef]
- Wakefield, B. J. Product Class 9: Isoxazoles. In Science of Synthesis; Georg Thieme Verlag: New York, 2002; Vol. 11, Chapter 9; pp. 229–288. [Google Scholar]
- Cicchi, S.; Cordero, F.M.; Giomi, D. Progress in Heterocyclic Chemistry; Gribble, G, Joule, J, Eds.; Pergamon Press: Oxford, 2003; Vol. 15, pp. 261–283. [Google Scholar]
- Sun, C.-M.; Lin, L.-G.; Yu, H.-J.; Cheng, C.-Y.; Xai, Y.-C.; Chu, C.-W.; Din, Y.-H.; Chau, Y.-P.; Don, M.-Y. Synthesis and Cytotoxic Activities of 4,5-Diarylisoxazoles. Bioorg. Med. Chem. Lett. 2007, 17, 1078–1081. [Google Scholar] [CrossRef]
- Chiacchio, U.; Balestrieri, B.; Macchi, B.; Iannazzo, D.; Piperno, A.; Rescifina, A.; Romeo, R.; Saglimbeni, M.; Sciortino, M.T.; Valveri, V.; Mastino, A.; Romeo, G. Synthesis of Phosphonated Carbocyclic 2'-Oxa-3'-aza-nucleosides: Novel Inhibitors of Reverse Transcriptase. J. Med. Chem. 2005, 48, 1389–1394. [Google Scholar] [CrossRef]
- Chiacchio, U.; Rescifina, A.; Romeo, G. Targets in Heterocyclic Systems: Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society of Chemistry: Rome, 1997; Vol. 1, pp. 225–275. [Google Scholar]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; John Wiley & Sons: New York, 1984. [Google Scholar]
- Wiley, R.H.; Wakefield, J. Studies on the Chemistry of Aspenwood. VII.1 Further Studies on the Ether Extractives of Commercial Aspen Spent Sulfite Liquor. J. Org. Chem. 1960, 26, 546–551. [Google Scholar] [CrossRef]
- Arcoria, A.; Ballistreri, F. P.; Spina, E.; Tomaselli, G. A.; Toscano, R. M. Kinetics of Mechanism of Oxidation of Organic Sulfides and Olefins by a Molybdenum Peroxopolyoxoanion. Gazz. Chim. Ital. 1990, 120, 309–313. [Google Scholar]
- Venturello, C.; D’Aloisio, R.; Bart, J.C.; Ricci, M. A New Peroxotungsten Heteropoly Anion with Special Oxidizing Properties: Synthesis and Structure of Tetrahexylammonium Tetra(diperoxotungsto)phosphate(3–). J. Mol. Catal. 1985, 32, 107–110. [Google Scholar] [CrossRef]
- Di Furia, F.; Modena, G. Mechanism of Oxygen Transfer from Peroxo Species. Pure Appl. Chem. 1982, 54, 1853–1866. [Google Scholar]
- Ballistreri, F. P.; Tomaselli, G. A.; Toscano, R. M.; Conte, V.; Di Furia, F. Application of the Thianthrene 5-Oxide Mechanistic Probe to Peroxometal Complexes. J. Am. Chem. Soc. 1991, 113, 6209–6212. [Google Scholar]
- Ballistreri, F. P.; Bazzo, A.; Tomaselli, G. A.; Toscano, R. M. Reactivity of Peroxopolyoxo Complexes. Oxidation of Thioethers, Alkenes, and Sulfoxides by Tetrahexylammonium Tetrakis(diperoxomolybdo)phosphate. J. Org. Chem. 1992, 57, 7074–7077. [Google Scholar] [CrossRef]
- Ishii, Y.; Yamawaki, K.; Ura, T.; Yamada, H.; Yoshida, T.; Ogawa, M. Hydrogen Peroxide Oxidation Catalyzed by Heteropoly Acids Combined with Cetylpyridinium Chloride. Epoxidation of Olefins and Allylic Alcohols, Ketonization of Alcohols and Diols, and Oxidative Cleavage of 1,2-Diols and Olefins. J. Org. Chem. 1988, 53, 3587–3593. [Google Scholar] [CrossRef]
- Cunico, R.F.; Bedell, L. J. Org. Chem. 1983, 48, 2780–2782. [CrossRef]
- Aversa, M.C.; Cum, G.; Crisafulli, M. NMR Spectra of 2-Isoxazolines. II. Cis-trans Isomers of 4,5-Disubstituted 2-Isoxazolines. Gazz. Chim. Ital. 1968, 98, 42–47. [Google Scholar]
- Christl, M.; Huisgen, R.; Sustmann, R. 1,3-Dipolar Cycloadditions. 71. Additions of Benzonitrile Oxide to α,β-Unsaturated Carboxylic Esters. Chem. Ber. 1973, 106, 3275–3290. [Google Scholar] Rahman, A.; Clapp, Leallyn, B. Thermal Decomposition of the Potassium Salts of Dinitroalkanes. J. Org. Chem. 1976, 41, 122–125. [Google Scholar]
- Hansen, T.V.; Wu, P.; Fokin, V.V. One-Pot Copper(I)-Catalyzed Synthesis of 3,5-Disubstituted Isoxazoles. J. Org. Chem. 2005, 70, 7761–7764. [Google Scholar] [CrossRef]
- Entry 7: Di Nunno, L.; Scilimati, A.; Vitale, P. 5-Hydroxy-3-phenyl-5-vinyl-2-isoxazoline and 3-Phenyl-5-vinylisoxazole: Synthesis and Reactivity. Tetrahedron 2005, 61, 11270–11278. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Xu, J.; Hamme, A.T. Isoxazoles from 1,1-Disubstituted Bromoalkenes. Tetrahedron Lett. 2007, 48, 1295–1298. [Google Scholar] [CrossRef]
- Leonardi, A.; Motta, G.; Riva, C.; Poggesi, E. Isoxazolecarboxamide Derivatives and their Adrenergic Antagonist Activity. PCT Int. Appl. WO 200102, 2001. [Google Scholar]
- Sample Availability: Samples of all compounds are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ballistreri, F.P.; Chiacchio, U.; Rescifina, A.; Tomaselli, G.; Toscano, R.M. Conversion of Oximes to Carbonyl Compounds by Triscetylpyridinium Tetrakis(oxodiperoxotungsto) Phosphate (PCWP)-mediated Oxidation with Hydrogen Peroxide. Molecules 2008, 13, 1230-1237. https://doi.org/10.3390/molecules13061230
Ballistreri FP, Chiacchio U, Rescifina A, Tomaselli G, Toscano RM. Conversion of Oximes to Carbonyl Compounds by Triscetylpyridinium Tetrakis(oxodiperoxotungsto) Phosphate (PCWP)-mediated Oxidation with Hydrogen Peroxide. Molecules. 2008; 13(6):1230-1237. https://doi.org/10.3390/molecules13061230
Chicago/Turabian StyleBallistreri, Francesco P., Ugo Chiacchio, Antonio Rescifina, Gaetano Tomaselli, and Rosa M. Toscano. 2008. "Conversion of Oximes to Carbonyl Compounds by Triscetylpyridinium Tetrakis(oxodiperoxotungsto) Phosphate (PCWP)-mediated Oxidation with Hydrogen Peroxide" Molecules 13, no. 6: 1230-1237. https://doi.org/10.3390/molecules13061230
APA StyleBallistreri, F. P., Chiacchio, U., Rescifina, A., Tomaselli, G., & Toscano, R. M. (2008). Conversion of Oximes to Carbonyl Compounds by Triscetylpyridinium Tetrakis(oxodiperoxotungsto) Phosphate (PCWP)-mediated Oxidation with Hydrogen Peroxide. Molecules, 13(6), 1230-1237. https://doi.org/10.3390/molecules13061230







