Results and Discussion
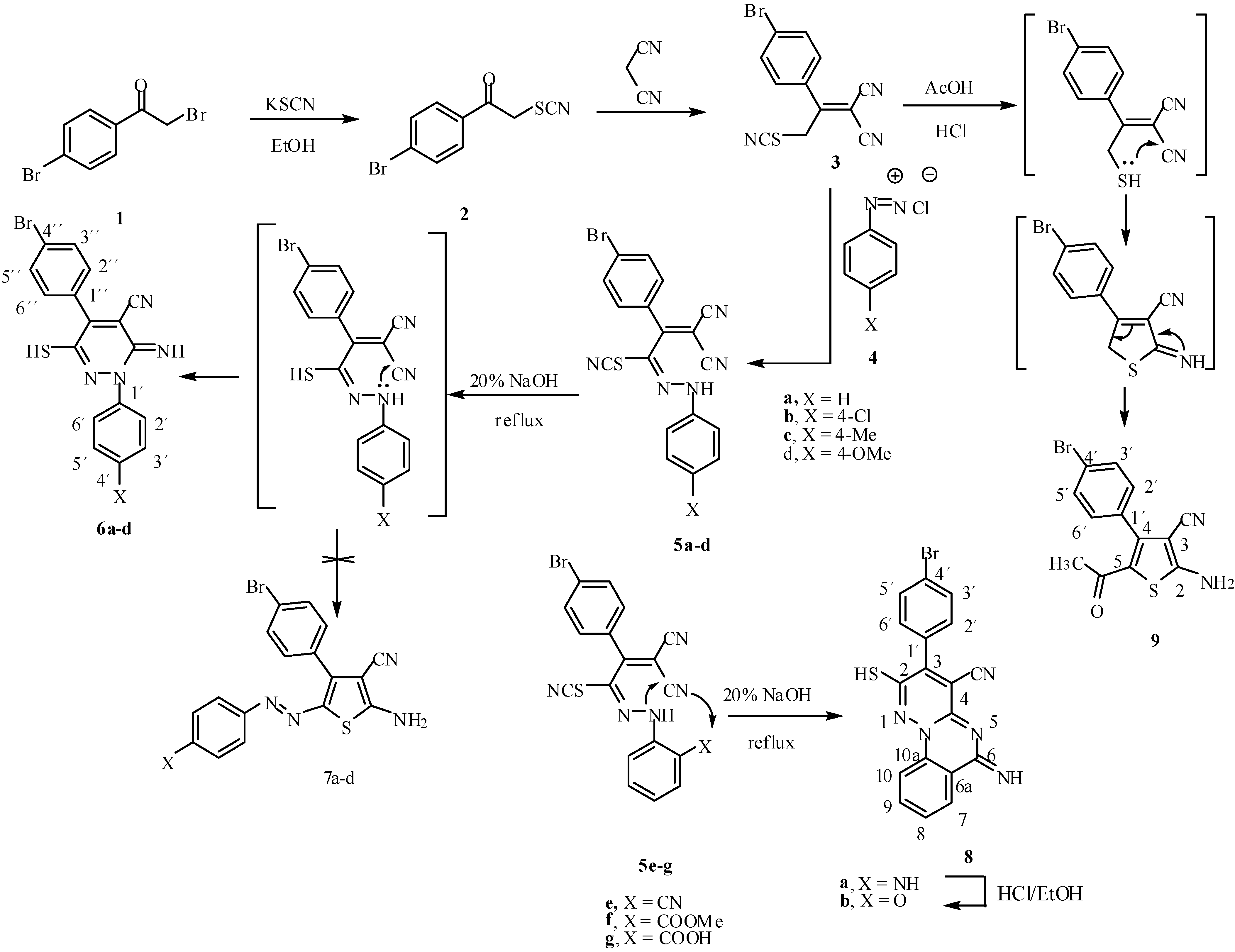
It has been found that reaction of compound
1 with potassium thiocyanate in ethanol produced the thiocyanate derivative
2 in 80% yield. Compound
2 condensed with malononitrile in ethanol in the presence of piperidine to afford the Knoevenagel condensation product
3 in 74 % yield. Compound
3 undergoes azo coupling reaction with diazotized aromatic amines to afford the arylhydrazone derivatives
5a-
g. Analytical and spectral data of these new arylhydrazone compounds were in complete agreement with the proposed structures. It had been previously reported [
17] that similar systems could be cyclized in acidic media, however, in our hands prolonged reflux under such acidic conditions did not produce the desired pyridazine derivatives
6a-
d, and we were only able to effect the cyclization of these arylhydrazone derivatives by refluxing in 20% ethanolic sodium hydroxide solution, although the SCN group was simultaneously hydrolysed to a SH group. Thus, compounds
5a-
d were cyclized to give the corresponding 3(
2H)-pyridazinimine derivatives
6a-
d, respectively (
Scheme 1). This cyclization is assumed to proceed via the hydrazonothiol intermediate, and the other possibility of cyclization to give the thiophene derivatives
7a-
d was readily ruled out on the basis of the
1H-NMR spectra of the products, which revealed the SH and NH signals at δ = 6.81 and 8.27 ppm, respectively, besides the aromatic protons at 7.52 ppm. In the case of compound
6d, the
13C-NMR and mass spectra were also in agreement with the proposed structure.
The arylhydrazone derivatives 5e underwent a cyclization reaction under conditions similar to those used for compounds 5a-d to afford the 2-mercapto-6H-pyridazino[1,6-a]quinazolin-6-imine derivative 8a, which was assumed to result from a double internal Michael addition of the NH to the neighboring CN group. The IR spectrum of 8a showed a broad NH absorption band at 3435, 3324 and a CN absorption band at 2224 cm-1. Its 1H-NMR spectrum revealed two singlets at 6.88 and 8.31 ppm, which were attributed to the SH and NH protons, respectively. The aromatic protons appeared at 7.72 ppm. The elemental analysis of 8a was in good agreement with the proposed structure.
Compounds 5f and 5g underwent a similar cyclization under the same conditions to produce the 2-mercapto-6H-pyridazino[1,6-a]quinazolin-6-one derivative 8b, apparently via loss of water or methanol, respectively. The IR spectrum of 8b showed absorption bands at 2214 and 1674 cm-1 corresponding to CN and C=O groups, respectively. The 1H-NMR spectrum of 8b revealed only one proton singlet at 6.80 ppm, which was attributed to the SH group, in addition to the aromatic protons at 8.23 ppm. Compound 8b could be obtained quantitatively from 8a upon refluxing the latter in ethanolic hydrochloric acid solution. The two products were matched by mixed m.p. and TLC analysis.
Scheme 1.
Reactivity of 2-(1-(4-bromophenyl)-2- thiocyanatoethylidene)malononitrile (3).
Scheme 1.
Reactivity of 2-(1-(4-bromophenyl)-2- thiocyanatoethylidene)malononitrile (3).
On the other hand, thiophene derivative
9 could be obtained in quantitative yield from compound
3 by refluxing in AcOH/HCl mixture for 3 h. The IR spectrum of
9 showed absorption bands at 3402, 2221, 1680 cm
-1, corresponding to NH
2, CN and C=O groups, respectively. The
1H-NMR spectrum of
9 revealed a singlet at 1.65 ppm (3H) and a singlet at 6.67 ppm (2H), which were attributed to the CH
3 and NH
2 groups, respectively, in addition to the four aromatic protons at 7.2 ppm. In the
13C-NMR of compound
9, 11 signals was found; the ones at 28.5, 114.4 and 186.1 ppm were attributed to the CH
3, CN and CO groups, respectively. A similar result was previously reported [
18]. From these data the reaction product could be formulated as the 5-acetyl-2-aminothiophene-3-carbonitrile derivative
9. The elemental analysis of
9 was in good agreement with the proposed structure (
Scheme 1).
Experimental
General
Melting points were measured on a Gallenkamp Electrothermal melting point apparatus and are uncorrected. IR spectra (KBr pellets) were recorded on a Pye Unicam SP 3-300 Spectrophotometer. NMR spectra were recorded in DMSO-d6 on Varian Gemini 200/300 MHz NMR spectrometers using tetramethylsilane (TMS) as an internal reference. Mass spectra were registered on a Shimadzu GCMS-QP 1000 Ex mass spectrometer at 70 eV. Elemental analyses were carried out at the Microanalytical Center of Cairo University.
1-(4-Bromophenyl)-2-thiocyanatoethanone (2)
To a solution of 1 (10 mmol) in EtOH (60 mL) was added KSCN (10 mmol). The reaction mixture was refluxed for 1.5 h. The mixture was then poured on ice-cold water, the solid collected by filtration and recrystallized from EtOH to give yellow crystals, 80% yield, mp. 148-149oC; IR (cm-1): 2175 (SCN), 1680 (CO); 1H-NMR δ: 5.07 (s, 2H, CH2), 7.77 (d, 2H, J = 8 Hz, Ar-H-3´,5´), 7.94 (d, 2H, J = 8 Hz, Ar-H-2´,6´); 13C-NMR δ: 41.56 (CH2), 112.75 (SCN), 128.51 (C-4´), 130.54 (C-2´, 6´), 132.03 (C-3´, 5´), 133.35 (C-1´), 190.67 (C-1); MS: 256 (M+79Br, 14%); 258 (M++2 81Br, 13%); Anal. Calcd. for C9H6BrNOS (256.12): C, 42.21; H, 2.36; N, 5.47. Found: C, 42.51; H, 2.65; N, 5.70.
2-(1-(4-Bromophenyl)-2-thiocyanatoethylidene)malononitrile (3)
A mixture of 2 (10 mmol) and malononitrile (10 mmol) was refluxed in EtOH (30 mL) in the presence of piperidine (2 mL) for 3 h, then left to cool at room temperature and the solid product was collected by filtration, washed with EtOH and recrystallized from EtOH to give green crystals, 74 % yield, mp. 258-260oC; IR (cm-1): 2210 (CN), 2175 (SCN); 1H-NMR δ: 5.41 (s, 2H, CH2), 7.64-7.72 (m, 4H, Ar-H); MS: 304 (M+79Br, 56%); 306 (M+ +2 81Br, 53%); Anal. Calcd. for C12H6BrN3S (304.17): C, 47.38; H, 1.99; N, 13.81. Found: C, 47.67; H, 2.31; N, 14.15.
General procedure for preparation of arylhydrazone derivatives 5a-g
To a stirred cold solution of 3 (10 mmol) and sodium acetate (10 g) in EtOH (50 mL) or pyridine (25 mL) was added dropwise over about 30 minutes a cold solution of a diazotized amine (aniline, 4-chloro-, 4-methyl-, 4-methoxyaniline, anthranilic acid, methyl anthranilate or anthranilonitrile, 10 mmol). The stirring was continued for 1h more. The coloured solids were collected by filtration, washed with cold water, and recrystallized from EtOH or 1:1 EtOH/DMF to afford 5a-g, respectively.
2-[1-(4-Bromophenyl)-2-(phenylhydrazono)-2-thiocyanatoethylidene] malononitrile (5a): Brown crystals (78%); mp. 204-206oC; IR (cm-1): 44308, 3310 (NH), 2214 (CN), 2178 (SCN); 1H-NMR δ: 7.34-7.60 (m, 5H, Ar-H), 7.82 (d, 2H, J = 8Hz, Ar-H), 7.95 (d, 2H, J = 8 Hz, Ar-H), 9.90 (s, 1H, NH).; MS: 408 (M+, 79Br, 38%); 410 (M++2, 81Br, 35%); Anal. Calcd. for C18H10BrN5S (408.28): C, 52.95; H, 2.47; N, 17.15. Found: C, 53.23; H, 2.71; N, 16.98.
2-[1-(4-Bromophenyl)-2-[(4-chlorophenyl)hydrazono]-2-thiocyanatoethylidene] malononitrile (5b): Yellow solid (81%); mp. 210-212oC; IR (cm-1): 4388, 3330 (NH), 2218 (CN), 2175 (SCN); 1H-NMR δ: 7.27-7.31 (m, 4H, Ar-H), 7.39-7.43 (m, 4H, Ar-H), 9.95 (bs, 1H, NH); Anal. Calcd. for C18H9BrClN5S (442.72): C, 48.83; H, 2.05; N, 15.82. Found: C, 49.12; H, 2.11; N, 16.17.
2-[1-(4-Bromophenyl)-2-thiocyanato-2-(p-tolylhydrazono)ethylidene] malononitrile (5c): Yellow solid (74%); mp. 218-219oC; IR (cm-1): 4395, 3318 (NH), 2221 (CN), 2177 (SCN); 1H-NMR δ: 2.27 (s, 3H, CH3), 7.10 (d, 2H, J = 9Hz, Ar-H), 7.47 (d, 2H, J = 9 Hz, Ar-H), 7.64 (d, 2H, J = 8 Hz, Ar-H), 7.92 (d, 2H, J = 8 Hz, Ar-H), 9.65 (s, 1H, NH); Anal. Calcd. for C19H12BrN5S (422.30): C, 54.04; H, 2.86; N, 16.58. Found: C, 54.34; H, 3.12; N, 16.44.
2-[1-(4-Bromophenyl)-2-[(4-methoxyphenyl)hydrazono]-2-thiocyanatoethylidene] malononitrile (5d): Yellow solid (74%); mp. 214-216oC; IR (cm-1): 4395, 3325 (NH), 2224 (CN), 2174 (SCN); 1H-NMR δ: 3.81 (s, 3H, OCH3), 7.02 (d, 2H, J = 9 Hz, Ar-H), 7.44 (d, 2H, J = 9 Hz, Ar-H), 7.49 (d, 2H, J = 8 Hz, Ar-H), 7.91 (d, 2H, J = 8 Hz, Ar-H), 11.05 (s, 1H, NH); Anal. Calcd. for C19H12BrN5OS (438.30): C, 52.07; H, 2.76; N, 15.98. Found: C, 52.37; H, 3.03; N, 16.24.
2-[1-(4-Bromophenyl)-2-[(2-cyanophenyl)hydrazono]-2-thiocyanatoethylidene] malononitrile (5e): Yellow solid (71%); mp. 205-207oC; IR (cm-1): 4385, 3330 (NH), 2220, 2212 (CN), 2177 (SCN); 1H-NMR δ: 7.49-7.65 (m, 4H, Ar-H), 7.74-8.30 (m, 4H, Ar-H), 10.95 (s, 1H, NH); Anal. Calcd. for C19H9BrN6S (433.29): C, 52.67; H, 2.09; N, 19.40. Found: C, 52.39; H, 2.33; N, 19.24.
2-[N'-[2-(4-Bromophenyl)-3,3-dicyano-1-thiocyanatoethylidene] hydrazono]benzoic acid methyl ester (5f): Yellow solid (77%); mp. 199-201oC; IR (cm-1): 3439, 3310 (NH), 2210 (CN), 2175 (SCN), 1668 (CO) cm-1; 1H-NMR δ: 2.62 (s, 3H, CH3), 7.27-7.49 (m, 4H, Ar-H), 7.65-8.30 (m, 4H, Ar-H), 11.10 (s, 1H, NH). MS: 465 (M+-1,79Br, 31%); 467 (M++1, 81Br, 32%); Anal. Calcd. for C20H12BrN5O2S (466.31): C, 51.51; H, 2.59; N, 15.02. Found: C, 51.72; H, 2.71; N, 15.30.
2-[ N'-[2-(4-bromophenyl)-3,3-dicyano-1-thiocyanatoallylidene]hydrazino)benzoic acid (5g): Yellow solid (70%); mp. 225-227oC; IR (cm-1): 3414, 3335 (NH), 2214 (CN), 2178 (SCN), 1685 (CO); 1H-NMR δ: 7.09-7.49 (m, 4H, Ar-H), 7.65-7.98 (m, 4H, Ar-H), 10.66 (s, 1H, NH), 14.03 (s, 1H, COOH); Anal. Calcd. for C19H10BrN5O2S (452.29): C, 50.46; H, 2.23; N, 15.48. Found: C, 50.81; H, 2.11; N, 15.61.
General procedure for preparation of compounds 6a-d and 8a,b
To a solution of each of 5a-g (10 mmol) in EtOH (25 mL) was added 20% aqueous NaOH solution (10 mL). The reaction mixture was refluxed for 2 h, then left to cool. The precipitated solid products formed were collected by filtration, washed with cold water, and recyrstallized from EtOH or 1:1 EtOH/DMF to afford 6a-d and 8a, b respectively.
5-(4-Bromophenyl)-3-imino-6-mercapto-2-phenyl-2,3-dihydropyridazine-4-carbonitrile (6a): Brown crystals (70%), mp. 233-235oC; IR (cm-1): 3404, 3318 (NH), 2206 (CN); 1H-NMR δ: 6.09 (s, 1H, SH), 7.14-7.42 (m, 2H, Ar-H), 7.52-7.60 (m, 3H, Ar-H), 7.75 (d, 2H, J = 8 Hz, Ar-H), 8.17 (d, 2H, J = 8 Hz, Ar-H), 8.33 (s, 1H, NH); MS: 383 (M+,79Br, 19%); 385 (M++2, 81Br, 18%); Anal. Calcd. for C17H11BrN4S (383.27): C, 53.27; H, 2.89; N, 14.62. Found: C, 53.33; H, 2.73; N, 14.53.
5-(4-Bromophenyl)-2-(4-chlorophenyl)-3-imino-6-mercapto-2,3-dihydropyridazine-4-carbonitrile (6b): Brown crystals (64%), mp. 263-265oC; IR (cm-1): 3400, 3322 (NH), 2209 (CN); 1H-NMR δ: 6.45 (s, 1H, SH), 7.54 (d, 2H, J = 8 Hz, Ar-H), 7.67 (d, 2H, J = 8 Hz, Ar-H), 7.89-8.10 (m, 4H, Ar-H), 8.29 (s, 1H, NH); Anal. Calcd. for C17H10BrClN4S (417.71): C, 48.88; H, 2.41; N, 13.41. Found: C, 48.69; H, 2.54; N, 13.77.
5-(4-Bromophenyl)-3-imino-6-mercapto-2-p-tolyl-2,3-dihydropyridazine-4-carbonitrile (6c): Brown crystals (64%), mp. 245-247oC; IR (cm-1): 3390, 3332 (NH), 2212 (CN); 1H-NMR δ: 2.34 (s, 3H, CH3), 5.87 (s, 1H, SH), 7.24 (d, 2H, J = 9 Hz, Ar-H), 7.44 (d, 2H, J = 9 Hz, Ar-H), 7.61 (d, 2H, J = 9 Hz, Ar-H), 8.26 (d, 2H, J = 9 Hz, Ar-H), 8.39 (s, 1H, NH); Anal. Calcd. for C18H13BrN4S (397.29): C, 54.42; H, 3.30; N, 14.10. Found: C, 54.75; H, 3.56; N, 14.34.
5-(4-bromophenyl)-3-imino-6-mercapto-2-(4-methoxyphenyl)-2,3dihydropyridazine-4-carbonitrile (6d): Brown crystals (69%), mp. 249-251oC; IR (cm-1): 3395, 3320 (NH), 2208 (CN); 1H-NMR δ: 3.65 (s, 3H, OCH3), 6.80 (s, 1H, SH), 7.26-7.87 (m, 8H, Ar-H), 8.27 (s, 1H, NH); 13C-NMR δ: 55.48 (OCH3), 89.81 (C-4), 112.43 (CN), 114.83 (C-3´5´), 115.93 (C-4´´), 118.59 (C-2´, 6´), 126.20 (C-2´´,6´´), 129.84 (C-3´´,5´´), 132.08 (C-1´´), 141.88 (C-1´), 148.97 (C-6), 155.42 (C-4´), 159.76 (C-5), 161.46 (C-3); Anal. Calcd. for C18H13BrN4OS (413.29): C, 52.31; H, 3.17; N, 13.56. Found: C, 52.45; H, 3.28; N, 13.84.
3-(4-Bromophenyl)-6-imino-2-mercapto-6H-pyridazino[1,6-a]quinazoline-4-carbonitrile (8a): Dark brown solid (59%), mp. 314-316oC; IR (cm-1): 3435 (NH), 2224 (CN); 1H-NMR δ: 6.88 (s, 1H, SH), 7.20 (d, 1H, J = 8 Hz, Ar-H10), 7.55-7.58 (m, 1H, Ar-H8), 7.68 (d, 1H, J = 8 Hz, Ar-H7), 7.77 (d, 2H, J = 9 Hz, Ar-H, 2´, 6´), 7.96-7.99 (m, 1H, Ar-H9), 8.24 (d, 2H, J = 9 Hz, Ar-H, 3´, 5´), 8.31 (s, 1H, NH); 13C-NMR δ: 105.46 (C-4), 116.20 (CN), 121.06 (C-10), 122.08 (C-6a), 125.53 (C-4´), 126.50 (C-7), 126.58 (C-2´, 6´), 128.97 (C-8), 129.98 (C-3´, 5´), 135.64 (C-9), 140.90 (C-1´), 141.22 (C-10a), 150.97 (C-2), 155.80 (C-3), 156.42 (C-4a), 161.95 (C-6); Anal. Calcd. for C18H10BrN5S (408.27): C, 52.95; H, 2.47; N, 17.15. Found: C, 52.79; H, 2.69; N, 17.34.
3-(4-Bromophenyl)-2-mercapto-6-oxo-6H-pyridazino[1,6-a]quinazoline-4-carbonitrile (8b): Violet crystals (77%), mp. 277-279oC; IR (cm-1): 2214 (CN), 1674 (C=O); 1H-NMR δ: 6.80 (s, 1H, SH), 7.61-7.66 (m, 1H, Ar-H10), 7.81-7.87 (m, 1H, Ar-H8), 7.91-7.99 (m, 1H, Ar-H9), 8.09 (d, 2H, J = 8 Hz, Ar-H, 2´,6´), 8.20-8.31 (m, 1H, Ar-H7), 8.86 (d, 2H, J = 9 Hz, Ar-H 3´,5´); Anal. Calcd. for C18H9BrN4OS (409.26): C, 52.83; H, 2.22; N, 13.69. Found: C, 53.12; H, 2.44; N, 14.04.
5-Acetyl-2-amino-4-(4-bromophenyl)thiophene-3-carbonitrile (9)
To a solution of 3 (50 mmol) in acetic acid (25 mL) was added concentrated HCl (10 mL) and the mixture was refluxed for 3 h. After cooling to room temperature, the reaction mixture was diluted with cold water and neutralized with ammonia. The solid formed was collected by filtration and recrystalized from EtOH/DMF (1:1) to afford 9. Yellow crystals (65%), mp. 237-239oC; IR (cm-1): 3402 (NH2), 2221 (CN), 1680 (C=O); 1H-NMR δ: 1.65 (s, 3H, CH3), 6.67 (s, 2H, NH2), 7.08 (d, 2H, J = 9 Hz, Ar-H), 7.39 (d, 2H, J = 9 Hz, Ar-H); 13C-NMR δ: 28.54 (CH3), 88.59 (C-3), 114.47 (C-3´,5´), 114.68 (CN), 118.20 (C-4´), 127.92 (C-2´,6´), 132.21 (C-5), 135.20 (C-1´), 147.45 (C-2), 159.37 (C-4), 186.15 (CO); MS: 321 (M+); Anal. Calcd. for C13H9BrN2OS (321.19): C, 48.61; H, 2.82; N, 8.72. Found: C, 48.87; H, 3.12; N, 8.89.