Aqueous Barbier Allylation of Aldehydes Mediated by Tin
Abstract
:Introduction
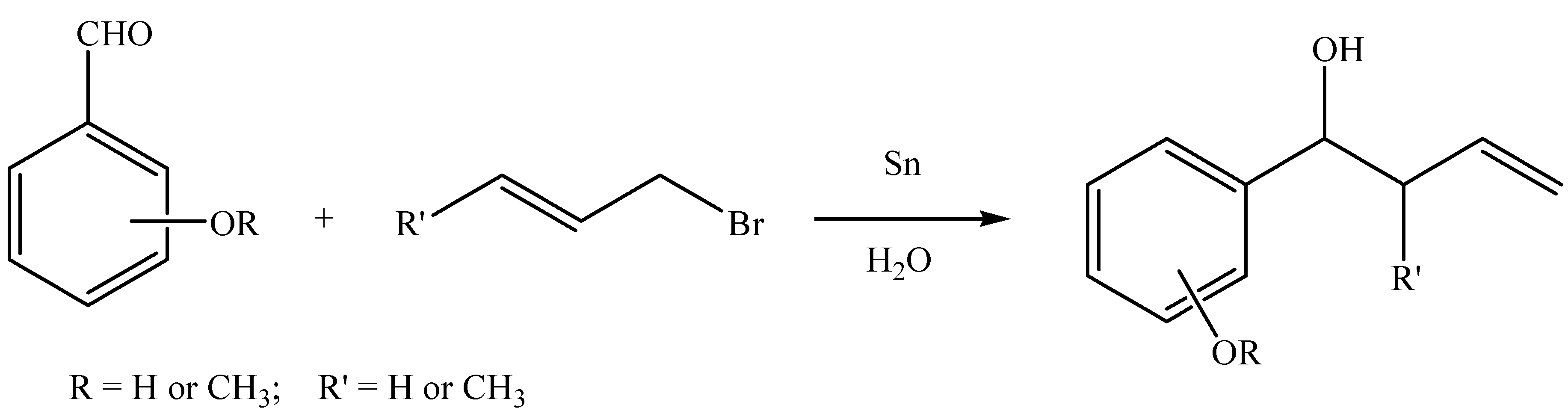
Results and Discussion
| Entry | Aldehyde | Halide | Salt added | Additive | Yield (%) | Syn/antia |
|---|---|---|---|---|---|---|
| 1 | 2-CH3O-C6H4CHO | C3H5Br | — | — | 73 | — |
| 2 | 2-CH3O-C6H4CHO | C3H5Br | K2HPO4 | — | 94 | — |
| 3 | 4-CH3O-C6H4CHO | C3H5Br | K2HPO4 | — | 80 | — |
| 4 | 3-CH3O-C6H4CHO | C3H5Br | K2HPO4 | — | 72 | — |
| 5 | 3-CH3O-C6H4CHO | C3H5Br | — | — | 84 | — |
| 6 | 2-HO-C6H4CHO | C3H5Br | — | — | 61 | — |
| 7 | 2-HO-C6H4CHO | C3H5Br | K2HPO4 | — | 85 | — |
| 8 | 3-HO-C6H4CHO | C3H5Br | K2HPO4b | — | 43 | — |
| 9 | 3-HO-C6H4CHO | C3H5Br | K2HPO4b | Pb(OAc)2 | 60 | — |
| 10 | 4-HO-C6H4CHO | C3H5Br | K2HPO4b | — | 25 | — |
| 11 | 4-HO-C6H4CHO | C3H5Br | K2HPO4b | Pb(OAc)2 | 71 | — |
| 12 | 2-CH3O-C6H4CHO | C4H7Br | K2HPO4 | — | 98 | 65:35 |
| 13 | 3-CH3O-C6H4CHO | C4H7Br | K2HPO4 | — | 98 | 50:50 |
| 14 | 4-CH3O-C6H4CHO | C4H7Br | — | — | 80 | 50:50 |
| 15 | 2-HO-C6H4CHO | C4H7Br | K2HPO4 | — | 84 | 70:30 |
| 16 | 3-HO-C6H4CHO | C4H7Br | K2HPO4 | — | 88 | 55:45 |
| 17 | 4-HO-C6H4CHO | C4H7Br | K2HPO4 | — | 72 | 57:43 |

| Entry | Aldehyde | Acid solution | Time (min) | Yield (%) |
| 1 a | 2-OCH3-C6H4CHO | HCl | 5 | 97 |
| 2 a | 2-OCH3-C6H4CHO | H2SO4 | 5 | 98 |
| 3 a | 2-OCH3-C6H4CHO | HNO3 | 5 | 94 |
| 4 a | 2-OCH3-C6H4CHO | CF3CO2H | 5 | 78 |
| 5 a | C6H5CHO | HCl | 5 | 93 |
| 6 a | 3-OCH3-C6H4CHO | HCl | 5 | 98 |
| 7 a | 2-OH-C6H4CHO | HCl | 5 | 98 |
| 8 a | 3-OH-C6H4CHO | HCl | 5 | 41 |
| 9a, b | 3-OH-C6H4CHO | HCl | 60 | 54 |
| 10 a | 3,4-(OCH3)2-C6H3CHO | HCl | 5 | 99 |
| 11 a | 3-OH-4-OCH3-C6H3CHO | HCl | 5 | 60 |
| 12 a | n-C6H13CHO | HCl | 30 | 90 |
| 13 a | (CH3)2CHCHO | HCl | 60 | 30 |
| 14c | C6H5CHO | HCl | 15 | 92 |
| 15 c | 2-OCH3-C6H4CHO | HCl | 15 | 95 |
| 16 c | 3-OCH3-C6H4CHO | HCl | 15 | 96 |
| 17 c | 4-OCH3-C6H4CHO | HCl | 15 | 86 |
| 18 c | 4-F-C6H4CHO | HCl | 15 | 88 |
| 19 c | 2-OH-C6H4CHO | HCl | 15 | 70 |
| 20 c | 2-OH-3-OCH3-C6H3CHO | HCl | 15 | 80 |
| 21 c | 3-OH-4-OCH3-C6H3CHO | HCl | 15 | 85 |
| 22 c | 3,4-(OCH3)2-C6H3CHO | HCl | 15 | 92 |
| 23 c | C6H5(CH2)2CHO | HCl | 15 | 65 |
| Entry | Aldehyde | Time (min) | Yield (%) | Syn/Antia |
| 1 b | C6H5CHO | 5 | 90 | 70 : 30 |
| 2 b | 2-OCH3-C6H4CHO | 5 | 93 | 75 : 25 |
| 3 b | 3-OCH3-C6H4CHO | 5 | 93 | 60 : 40 |
| 4 b | 4-OCH3-C6H4CHO | 5 | 74 | 65 : 35 |
| 5 b | 2-OH-C6H4CHO | 5 | 94 | 65 : 35 |
| 6 b | 3,4-(OCH3)2-C6H3CHO | 5 | 98 | 70 : 30 |
| 7 b | 3-OH-4-OCH3-C6H3CHO | 5 | 93 | 70 : 30 |
| 8 b | 4-F-C6H4CHO | 5 | 85 | 65 : 35 |
| 9 b | n-C6H13CHO | 60 | 93 | 60 : 40 |
| 10 c | C6H5CHO | 15 | 98 | 70 : 30 |
| 11 c | 4-F-C6H4CHO | 15 | 93 | 65 : 35 |
| 12 c | 2-OCH3-C6H4CHO | 15 | 95 | 75 : 25 |
| 13 c | 2-OH-C6H4CHO | 15 | 80 | 65 : 35 |
| 14 c | C6H5(CH2)2CHO | 30 | 90 | 75 : 25 |
| 15 b | 2-OCH3-C6H4CHO | 60 | 91 | 60 : 40 |
| 16 b | 2-OCH3-C6H4CHO | 240 | 67 | 50 : 50 |
| Entry | Reagent | EA (kcal mol-1) | E (V) |
|---|---|---|---|
| 1 | C6H5CHO | 24.7 | –1.36 |
| 2 | 2-HO-C6H4CHO | 28.8 | –1.48 |
| 3 | 3-HO-C6H4CHO | 27.8 | –1.68 |
| 4 | 4-HO-C6H4CHO | 26.1 | –2.14 |
| 5 | 2-CH3O-C6H4CHO | 27.4 | –1.55 |
| 6 | 3-CH3O-C6H4CHO | 28.3 | –1.50 |
| 7 | 4-CH3O-C6H4CHO | 26.3 | –1.64 |
| 8 | CH2=CHCH2Br | 3.21 | –1.73a |
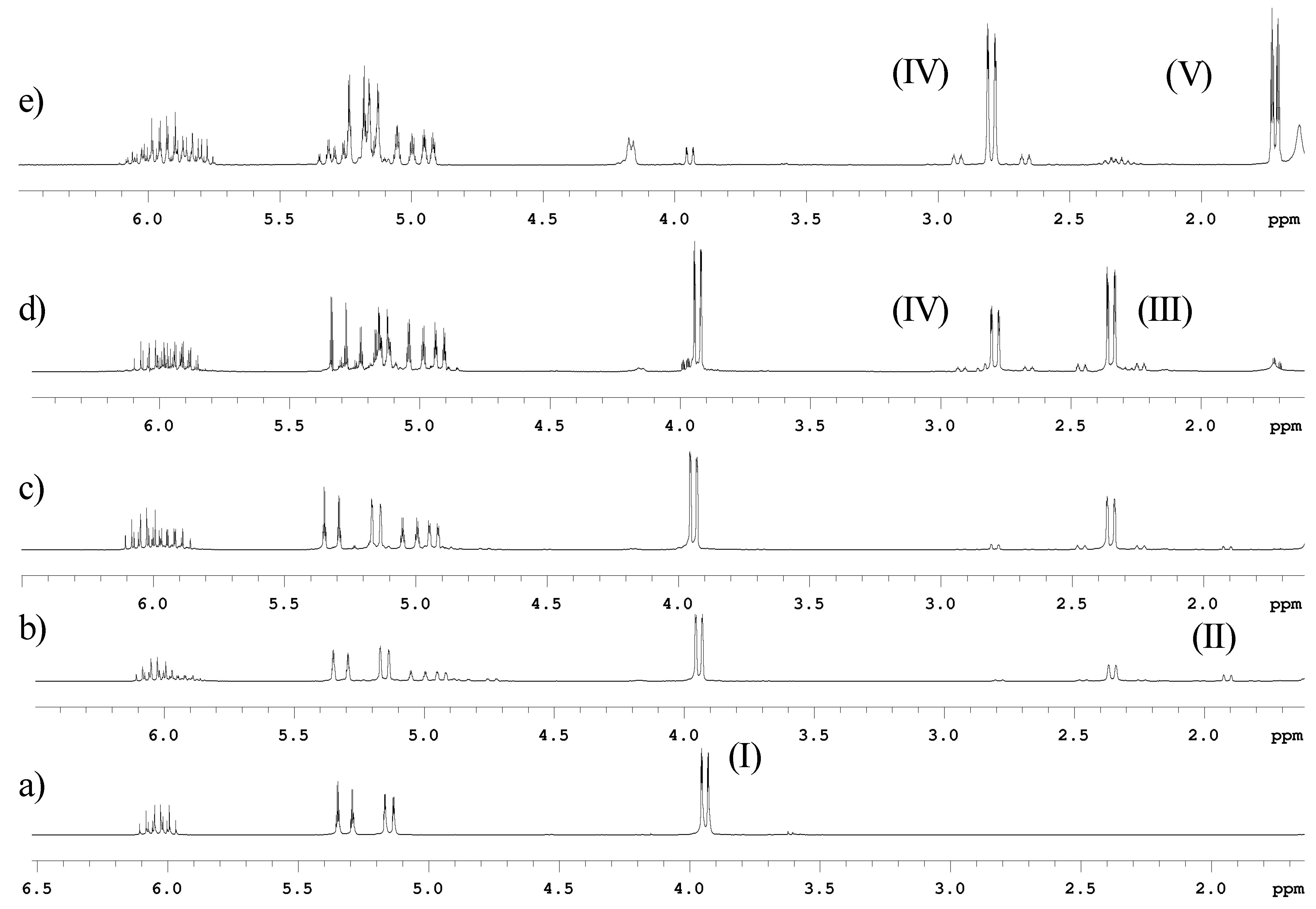

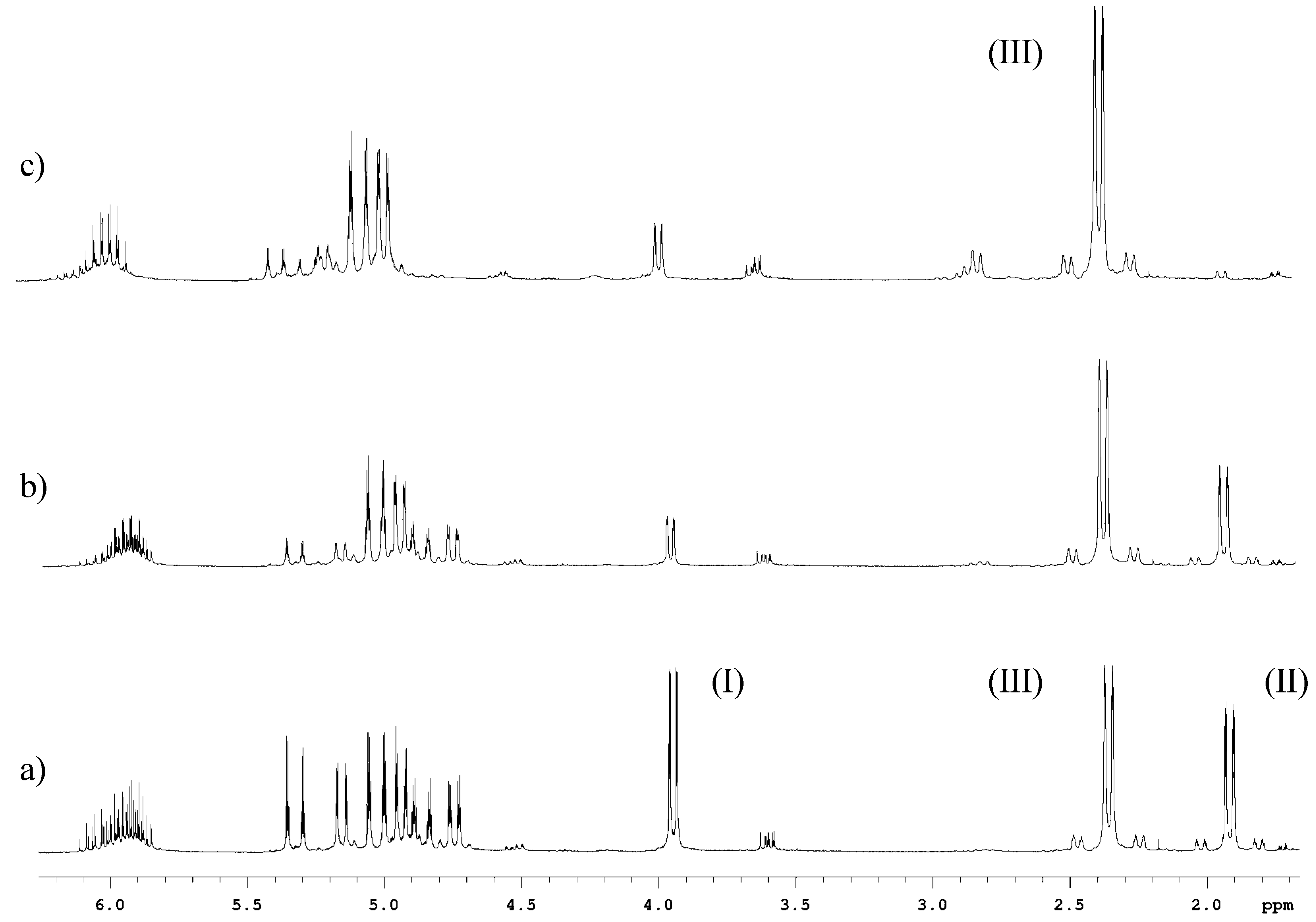
Conclusions
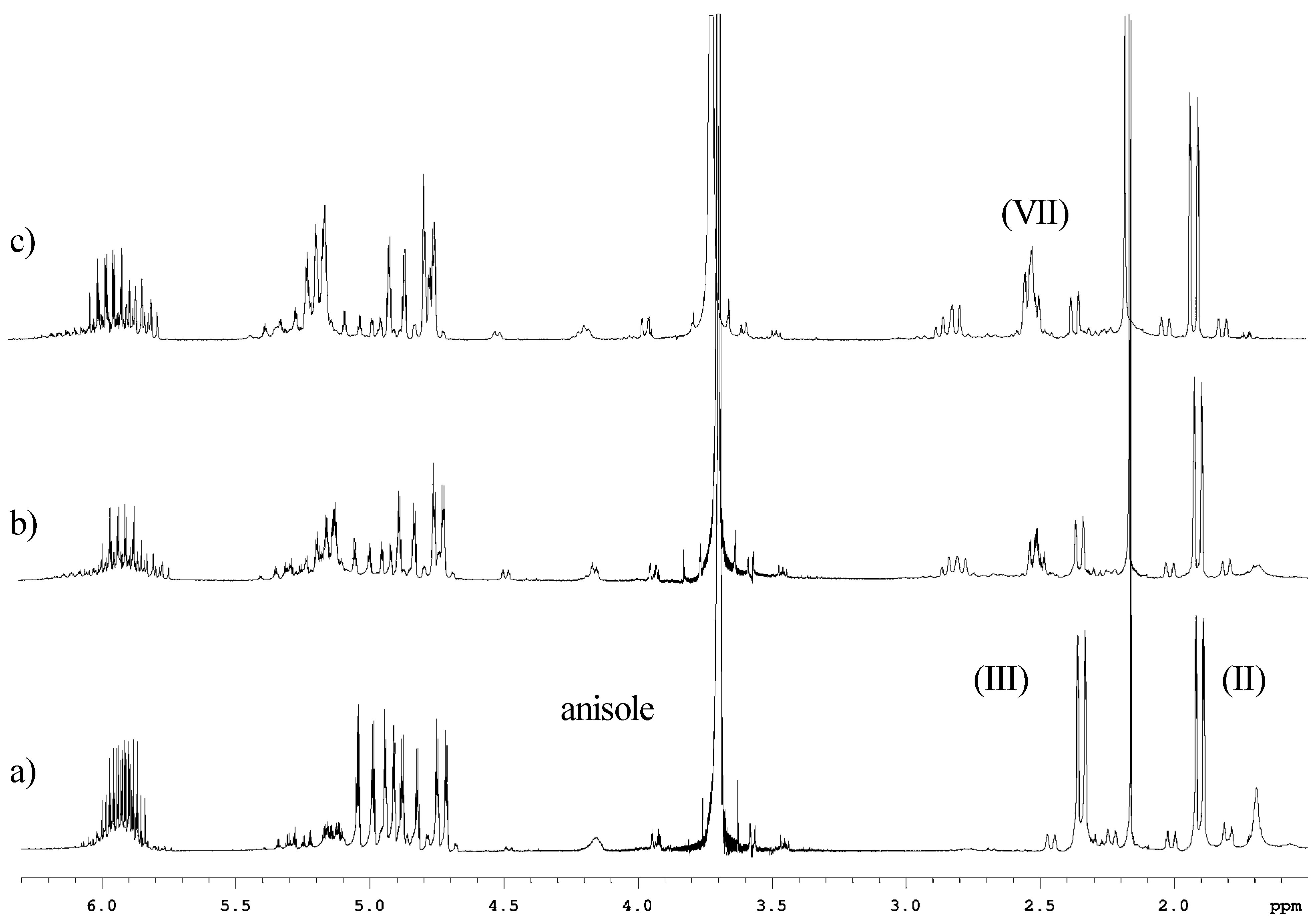
Experimental
General
General procedure for the Barbier reaction mediated by tin under basic conditions
General procedure for the Barbier reaction mediated by tin under acidic conditions
Procedure for the organotin intermediate formation
Reaction between 2-methoxybenzaldehyde and tetrallyltin
Measurement of the reduction potential and electron affinity calculations
Acknowledgments
References and Notes
- Li, C. J. Aqueous Barbier-Grignard Type Reaction: Scope, Mechanism, and Synthetic Applications. Tetrahedron 1996, 52, 5643–5668. [Google Scholar] [CrossRef] Tan, X. H.; Hou, Y. Q.; Huang, C.; Liu, L.; Guo, Q. X. SnCl2-mediated carbonyl allylation in fully aqueous media. Tetrahedron 2004, 60, 6129–6136. [Google Scholar] Tan, K. T.; Chng, S. S.; Cheng, H. S.; Loh, T. P. Development of a Highly α-Regioselective Metal-Mediated Allylation Reaction in Aqueous Media: New Mechanistic Proposal for the Origin of α-Homoallylic Alcohols. J. Am. Chem. Soc. 2003, 125, 2958–2963. [Google Scholar] Nicolau, K. C.; Ninchovic, S.; Sarabia, F.; Vourloumis, D.; He, Y.; Vallberg, H.; Finlay, M. R. V.; Yang, Z. Total Syntheses of Epothilones A and B via a Macrolactonization-Based Strategy. J. Am. Chem. Soc. 1997, 119, 7974–7991. [Google Scholar]
- Li, C. J. Organic Reactions in Aqueous Media with a Focus on Carbon-Carbon Bond Formations: A Decade Update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar] Li, C. J.; Chan, T. H. Organic Reactions in Aqueous Media; John Wiley & Sons: New York, 1997. [Google Scholar]
- Anastas, P. T.; Kirchhoff, M. M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Petrier, C.; Luche, J. L. Allylzinc reagents additions in aqueous media. J. Org. Chem. 1985, 50, 910–912. [Google Scholar] [CrossRef] Marton, D.; Stivanello, D.; Tagliavini, G. Study of the Allylation of Aldehydes with Allyl Halides in Cosolvent/Water(Salt)/Zn and in Cosolvent/Water(Salt)/Zn/Haloorganotin Media. J. Org. Chem. 1996, 61, 2731–2737. [Google Scholar] Chung, W.; Higashiya, S.; Oba, Y.; Welch, J. Indium and zinc-mediated allylation of difluoroacetyltrialkylsilanes in aqueous media. Tetrahedron 2003, 59, 10031–10036. [Google Scholar] Lu, W. S.; Chan, T. H. Organometallic Reactions in Aqueous Media. Indium and Zinc-Mediated Allylation of Sulfonimines. J. Org. Chem. 2000, 65, 8589–8594. [Google Scholar]
- Nokami, J.; Otera, J.; Sudo, T.; Okawara, R. Allylation of aldehydes and ketones in the presence of water by allylic bromides, metallic tin, and aluminum. Organometallics 1983, 2, 191–193. [Google Scholar] [CrossRef] Zhou, J. Y.; Chen, Z. G.; Wu, S. H. Tin-promoted stereocontrolled intramolecular allylation of carbonyl-compounds - a facile and stereoselective method for ring construction. Chem. Commun. 1994, 2783–2784. [Google Scholar]
- Chan, T. H.; Li, C. J.; Lee, M. C.; Wei, Z. Y. 1993 Ru-Lemieux-Award-Lecture - Organometallic-type Reactions in Aqueous-Media - A New Challenge in Organic-Synthesis. Can. J. Chem. 1994, 72, 1181–1192. [Google Scholar] [CrossRef] Paquette, L. A.; Mitzel, T. M. Addition of Allylindium Reagents to Aldehydes Substituted at Cα or Cβ with Heteroatomic Functional Groups. Analysis of the Modulation in Diastereoselectivity Attainable in Aqueous, Organic, and Mixed Solvent Systems. J. Am. Chem. Soc. 1996, 118, 1931–1937. [Google Scholar] Chan, Y. H.; Yang, Y. J. Indium-Mediated Organometallic Reactions in Aqueous Media: The Nature of the Allylindium Intermediate. J. Am. Chem. Soc. 1999, 121, 3228–3229. [Google Scholar]
- Li, C. J.; Meng, Y.; Yi, X. H. Manganese-Mediated Carbon-Carbon Bond Formation in Aqueous Media: Chemoselective Allylation and Pinacol Coupling of Aryl Aldehydes. J. Org. Chem. 1998, 63, 7498–7504. [Google Scholar] [CrossRef]
- Miyamoto, H.; Daikawa, N.; Tanaka, K. Carbon-carbon bond formation using bismuth in a water medium. Tetrahedron Lett. 2003, 44, 6963–6964. [Google Scholar] [CrossRef] Chan, T. C.; Lau, C. P.; Chan, T. H. Iron–mediated allylation of aryl aldehydes in aqueous media. Tetrahedron Lett. 2004, 45, 4189–4191. [Google Scholar]
- Schimd, W.; Whitesides, G. M. Carbon-carbon bond formation in aqueous ethanol: diastereoselective transformation of unprotected carbohydrates to higher carbon sugars using allyl bromide and tin metal. J. Am. Chem. Soc. 1991, 113, 6674–6675. [Google Scholar] [CrossRef]
- Yasuda, M.; Fujibayashi, T.; Baba, A. Allylation of Carbonyl Compounds Bearing a Hydroxyl Group by Tetraallyltin: Highly Stereoselective Allylation in a Chelation-Controlled Manner. J. Org. Chem. 1998, 63, 6401–6404. [Google Scholar] [CrossRef]
- Wang, Z.; Zha, Z.; Zhou, C. Application of Tin and Nanometer Tin in Allylation of Carbonyl Compounds in Tap Water. Org. Lett. 2002, 4, 1683–1685. [Google Scholar] [CrossRef]
- Zha, Z.; Qiao, S. Q.; Jiang, J.; Wang, Y.; Miao, Q.; Wang, Z. Barbier-type reaction mediated with tin nano-particles in water. Tetrahedron 2005, 61, 2521–2527. [Google Scholar] [CrossRef]
- Zha, Z.; Hui, A.; Zhou, Y.; Miao, Q.; Wang, Z.; Zhang, H. A Recyclable Electrochemical Allylation in Water. Org. Lett. 2005, 7, 1903–1905. [Google Scholar] [CrossRef]
- Takahara, J. P.; Masuyama, Y.; Kumsu, Y. Palladium-catalyzed carbonyl allylation by allylic alcohols with stannous chloride. J. Am. Chem. Soc. 1992, 114, 2577–2586. [Google Scholar] [CrossRef] Bieber, L. W.; Malvestiti, I.; Storch, E. C. Reformatsky Reaction in Water: Evidence for a Radical Chain Process. J. Org. Chem. 1997, 62, 9061–9064. [Google Scholar] Lannou, M. I.; Hélion, F.; Namy, J. L. Catalytic Barbier-type reactions of lactones and esters mediated by the Mischmetall/SmI2(cat.) system or the Mischmetall/[SmI2/NiI2(cat.)](cat.). Tetrahedron Lett. 2002, 43, 8007–8010. [Google Scholar]
- Wada, M.; Ohki, H.; Akiba, K. –Y. Bismuth(III) Chloride Aluminum-promoted Allylation of Aldehydes to Homoallylic Alcohols in Aqueous Solvent. Bull. Chem. Soc. Jpn. 1990, 1738–1747. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yatagai, H.; Naruta, Y.; Maruyama, K. Erythro-selective addition of crotyl-trialkyltins to aldehydes regardless of the geometry of the crotyl unit. Stereoselection independent of the stereochemistry of precursors. J. Am. Chem. Soc. 1980, 102, 7107–7109. [Google Scholar] Zha, Z.; Quiao, S. Q.; Jiang, J.; Wang, Y.; Miao, Q.; Wang, Z. Barbier-type reaction mediated with tin nano-particles in water. Tetrahedron 2005, 61, 2521–2527. [Google Scholar]
- Paquette, L.A.; Mitzel, T.M. Comparative diastereoselectivity analysis of crotylindium and 3-bromoallylindium additions to α-oxy aldehydes in aqueous and nonaqueous solvent systems. J. Org. Chem. 1996, 61, 8799–8804. [Google Scholar] [CrossRef] Chung, W.; Chan, T. H. Indium-mediated organometallic reactions in aqueous media. Stereoselectivity in crotylation of sulfonimines bearing a proximal chelating group. J. Org. Chem. 2001, 66, 3467–3473. [Google Scholar]
- Furlani, D.; Marton, D.; Tagliavini, G.; Zordan, M. Hydrated sigma-bonded organometallic cations in organic-synthesis. 1. allyl-stannation, crotyl-stannation, 1-methylallyl-stannation, cyclohex-2-enyl-stannation, and cinnamyl-stannation of carbonyl-compounds in water. J. Organomet. Chem. 1988, 341, 345–356. [Google Scholar] [CrossRef]
- Sinha, P.; Roy, S. Barbier Reaction in the Regime of Metal Oxide: Carbonyl Allylation over SnO/Cu2O and Surface Diagnostics. Organometallics 2004, 23, 67–71. [Google Scholar] [CrossRef]
- Crosby, S. R.; Harding, J. R.; King, C. D.; Parker, G. D.; Willis, C. L. Oxonia-Cope Rearrangement and Side-Chain Exchange in the Prins Cyclization. Org. Lett. 2002, 4, 577–580. [Google Scholar] [CrossRef]
- Jasti, R.; Rychnovsky, S. D. Racemization in Prins Cyclization Reactions. J. Am. Chem. Soc. 2006, 128, 13640–13648. [Google Scholar] [CrossRef]
- Moyano, A.; Pericàs, M. A.; Riera, A.; Luche, J. L. A theoretical study of the Barbier reaction. Tetrahedron Lett. 1990, 31, 7619–7622. [Google Scholar] [CrossRef]
- Bard, A.J; Merz, A. Electrochemical reduction of allyl halides in nonaqueous solvents - a reinvestigation. J. Am. Chem. Soc. 1979, 101, 2959–2965. [Google Scholar] [CrossRef]
- Beckwith, A. L. J.; Bowry, V. W.; Ingold, K. U. Kinetics of nitroxide radical trapping. 1. Solvent effects. J. Am. Chem. Soc. 1992, 114, 4983–4992. [Google Scholar] [CrossRef] Bowry, V. W.; Ingold, K. U. Kinetics of nitroxide radical trapping. 2. Structural effects. J. Am. Chem. Soc. 1992, 114, 4992–4996. [Google Scholar]
- Marshall, J. A.; Hinkle, K. W. Synthesis of anti-Homoallylic Alcohols and Monoprotected 1,2-Diols through InCl3-Promoted Addition of Allylic Stannanes to Aldehydes. J. Org. Chem. 1995, 60, 1920–1921. [Google Scholar] [CrossRef]
- Chan, T. H.; Yang, Y.; Li, C. J. Organometallic Reactions in Aqueous Media. The Nature of the Organotin Intermediate in the Tin-Mediated Allylation of Carbonyl Compounds. J. Org. Chem. 1999, 64, 4452–4455. [Google Scholar] [CrossRef]
- Chung, L. W.; Chan, T. H.; Wu, Y.D. Theoretical study of Intrinsic reactivities of various allylmetals toward carbonyl and water. Organometallics 2005, 24, 1598–1607. [Google Scholar] [CrossRef]
- Taylor, M. J.; Coddington, J. M. The constitution of aqueous tin(IV) chloride and bromide solutions and solvent extracts studied by 119Sn NMR and vibrational spectroscopy. Polyhedron 1992, 11, 1531–1544. [Google Scholar] [CrossRef]
- Thoonen, S. H. L.; Deelman, B. J.; Koten, G. Synthetic aspects of tetraorganotins and organotin(IV) halides. J. Organomet. Chem. 2004, 689, 2145–2157. [Google Scholar] [CrossRef]
- Naruta, Y.; Nishigaichi, Y.; Maruyam, K. NMR study on transmetalation reaction between allyltins and SnCl4. Tetrahedron 1989, 45, 1067–1078. [Google Scholar] [CrossRef]
- Petrosyan, V.S. NMR-spectra and structures of organotin compounds. Prog. NMR Spectrosc. 1977, 11, 115–148. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Guimarães, R.L.; Lima, D.J.P.; Barros, M.E.S.B.; Cavalcanti, L.N.; Hallwass, F.; Navarro, M.; Bieber, L.W.; Malvestiti, I. Aqueous Barbier Allylation of Aldehydes Mediated by Tin. Molecules 2007, 12, 2089-2105. https://doi.org/10.3390/12092089
Guimarães RL, Lima DJP, Barros MESB, Cavalcanti LN, Hallwass F, Navarro M, Bieber LW, Malvestiti I. Aqueous Barbier Allylation of Aldehydes Mediated by Tin. Molecules. 2007; 12(9):2089-2105. https://doi.org/10.3390/12092089
Chicago/Turabian StyleGuimarães, Ricardo L., Dimas J. P. Lima, Maria Ester S. B. Barros, Lívia N. Cavalcanti, Fernando Hallwass, Marcelo Navarro, Lothar W. Bieber, and Ivani Malvestiti. 2007. "Aqueous Barbier Allylation of Aldehydes Mediated by Tin" Molecules 12, no. 9: 2089-2105. https://doi.org/10.3390/12092089
APA StyleGuimarães, R. L., Lima, D. J. P., Barros, M. E. S. B., Cavalcanti, L. N., Hallwass, F., Navarro, M., Bieber, L. W., & Malvestiti, I. (2007). Aqueous Barbier Allylation of Aldehydes Mediated by Tin. Molecules, 12(9), 2089-2105. https://doi.org/10.3390/12092089






