Aqueous Ti(IV)-Catalyzed Diels-Alder Reaction
Abstract
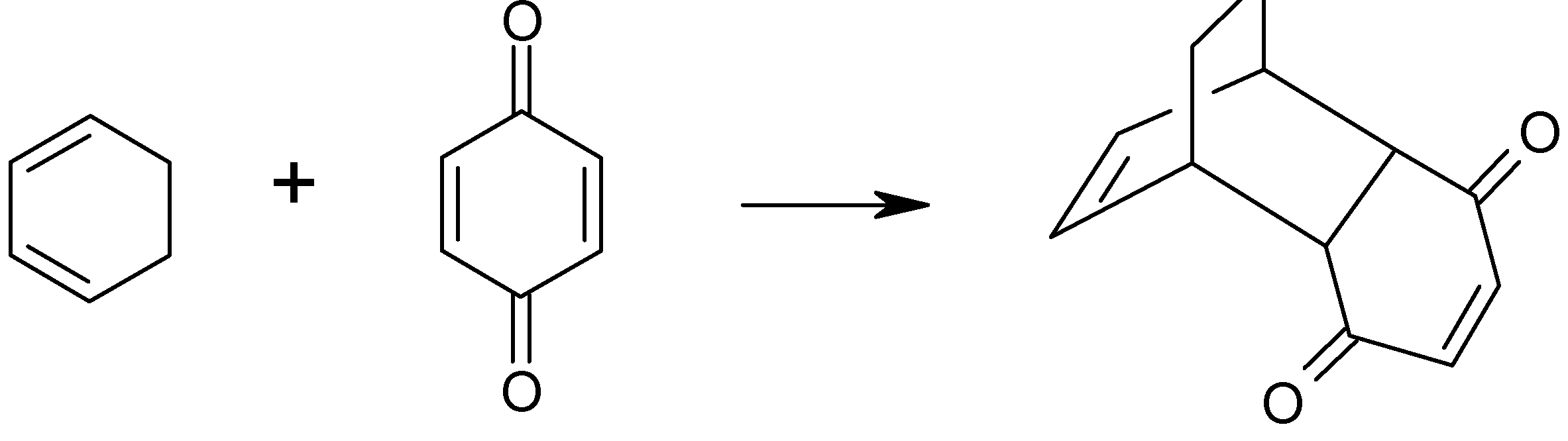
:Introduction
Results and Discussion
| [Catalyst] | % Yield* (std deviation) |
|---|---|
| 0 mole % | 61% (±1.5%) |
| 5 mole % | 83% (±1.5%) |

Conclusions
Experimental
General
Typical Ti(IV)-catalyzed reaction
References and Notes
- Fringuelli, F.; Piermatti, O.; Pizzo, F.; Vaccaro, L. Recent Advances in Lewis Acid Catalyzed Diels-Alder Reactions in Water. Eur. J. Org. Chem. 2001, 439–455. [Google Scholar]
- Lindstrom, U. F. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef]
- For reviews: Whitesides, C.H. Enzymes in Organic Chemistry; Pergamon Press: New York, 1994. [Google Scholar] Anostas, P.T.; Williamson, T.C. (Eds.) Green Chemistry. Designing Chemistry for the Environment; ACS Publications: Washington, D.C., 1996. Schlosser, M. (Ed.) Organometallics in Synthesis. A Manual; Wiley: New York, 1996. Komiya, S. Synthesis of Organometallic Compounds. A Practical Guide; Wiley: New York, 1997. [Google Scholar] Li, C.J.; Chang, T.H. Organic Reactions in Aqueous Media; Wiley: New York, 1997. [Google Scholar] Grieco, P.A. (Ed.) Organic Synthesis in Water; Blackie Acad. Professional Publishers: London, 1998.
- Rideout, D.C.; Breslow, R. Hydrophobic Acceleration of Diels-Alder Reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Rispens, T.; Engberts, J. B. F. N. Efficient Catalysis of a Diels-Alder Reaction by Metallo-Vesicles in Aqueous Solution. Org. Lett. 2001, 3, 941–943. [Google Scholar] [CrossRef] Otto, S.; Engberts, J. B. F. N. Efficient Catalysis of a Diels-Alder Reaction by Metallo-Vesicles in Aqueous Solution. J. Am. Chem. Soc. 1999, 121, 6798–6806. [Google Scholar] [CrossRef] Loh, T.-P.; Pei, J.; Lin, M. Indium Trichloride (InCl3) Catalyzed Diels-Alder Reaction in Water. Chem. Commun. 1996, 2315–2316. [Google Scholar] Otto, S.; Bertoncin, F.; Engberts, J. B. F. N. Lewis Acid Catalysis of a Diels-Alder Reaction in Water. J. Am. Chem. Soc. 1996, 118, 7702–7707. [Google Scholar] [CrossRef] Otto, S.; Engberts, J. B. F. N. Lewis Acid Catalysis of a Diels-Alder Reaction in Water. Tetrahedron Lett. 1995, 36, 2645–2648. [Google Scholar] [CrossRef]
- Bosnich, B. Transition-Metal-Based Lewis Acid Catalysts. Aldrichim. Acta 1998, 31, 76–83. [Google Scholar]
- Odenkirk, W.; Rheingold, A.L.; Bosnich, B. Homogeneous Catalysis: A Ruthenium-Based Lewis-Acid Catalyst for the Diels-Alder Reaction. J. Am. Chem. Soc. 1992, 114, 6392–6398. [Google Scholar] [CrossRef]
- Sunakawa, T.; Kuroda, C. Substrate Dependence in Aqueous Diels-Alder Reactions of Cyclohexadiene Derivatives with 1,4-Benzoquinone. Molecules 2005, 10, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Grimme, W.; Wortmann, J.; Frowein, D.; Lex, J.; Chen, G.; Gleiter, R. Laticyclic Conjugated Double Bonds Within the Framework of Oligocondensed Bicyclo[2.2.2]octenes. J. Chem. Soc., Perkin Trans. 2 1998, 1893–1900. [Google Scholar]
- Sample Availability: Samples of the compounds are available from author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Litz, K.E. Aqueous Ti(IV)-Catalyzed Diels-Alder Reaction. Molecules 2007, 12, 1674-1678. https://doi.org/10.3390/12081674
Litz KE. Aqueous Ti(IV)-Catalyzed Diels-Alder Reaction. Molecules. 2007; 12(8):1674-1678. https://doi.org/10.3390/12081674
Chicago/Turabian StyleLitz, Kyle E. 2007. "Aqueous Ti(IV)-Catalyzed Diels-Alder Reaction" Molecules 12, no. 8: 1674-1678. https://doi.org/10.3390/12081674




