Heterocycles [h]Fused onto 4-Oxoquinoline-3-Carboxylic Acid, Part IV. Convenient Synthesis of Substituted Hexahydro [1,4]Thiazepino[2,3-h]quinoline-9-carboxylic Acid and Its Tetrahydroquino[7,8-b]benzothiazepine Homolog
Abstract
:Introduction
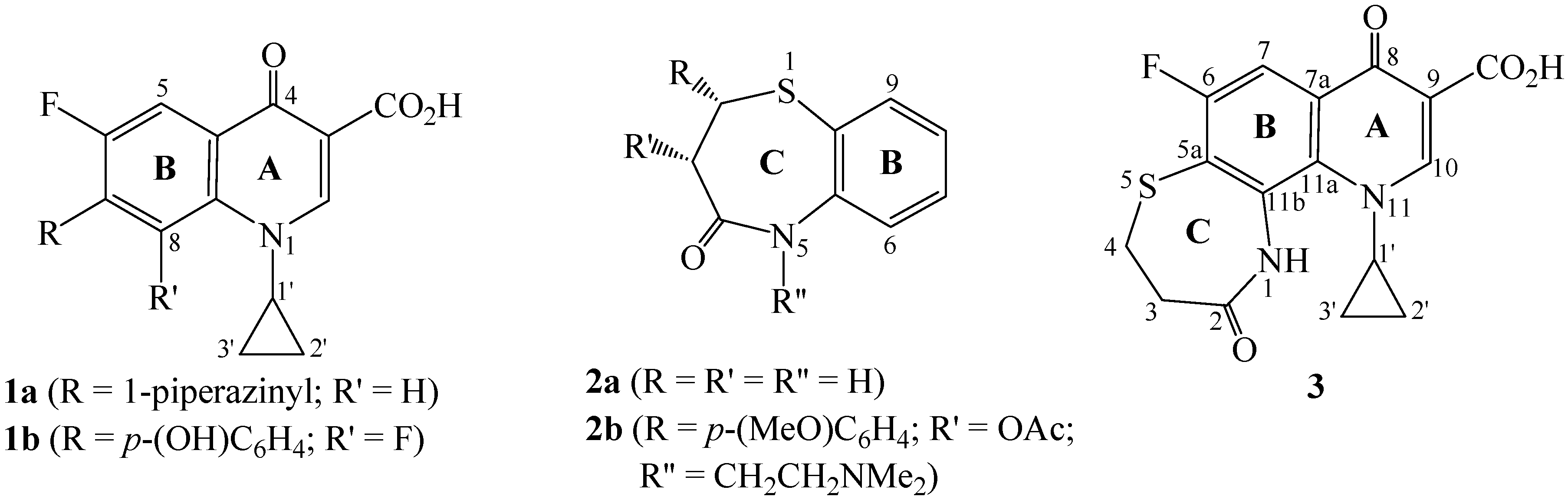
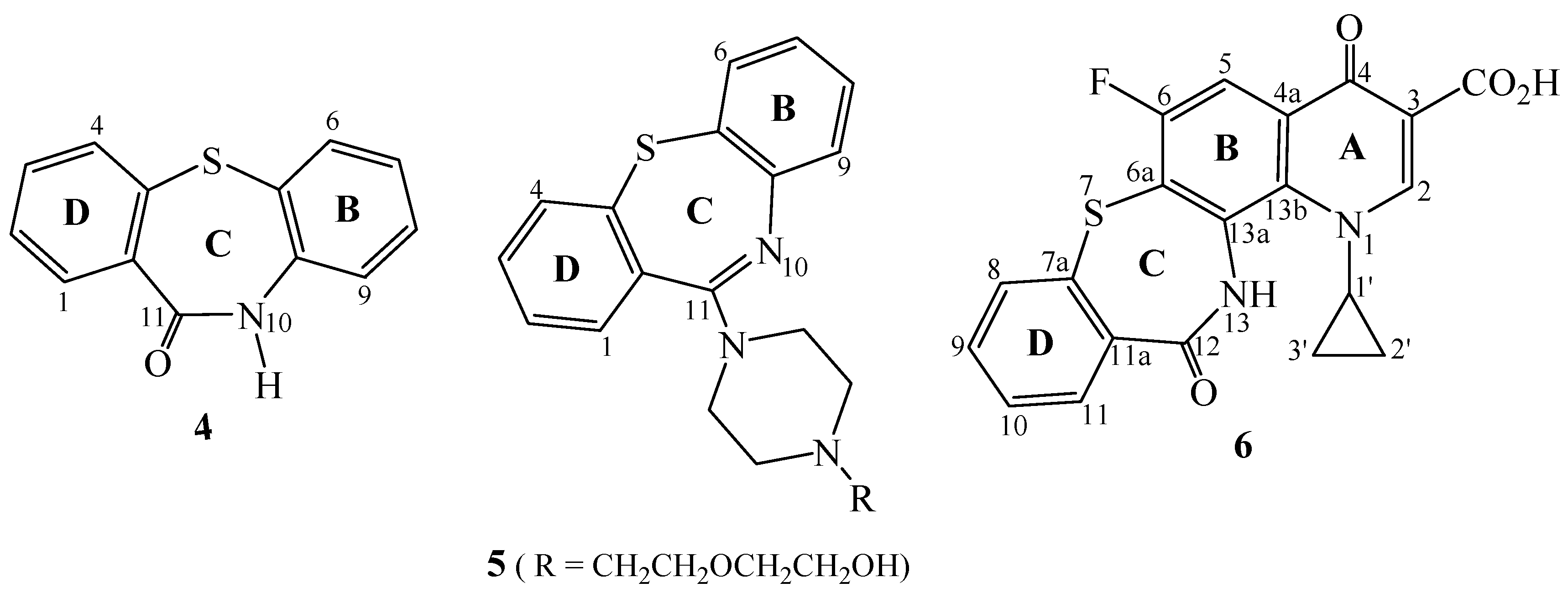
Results and Discussion

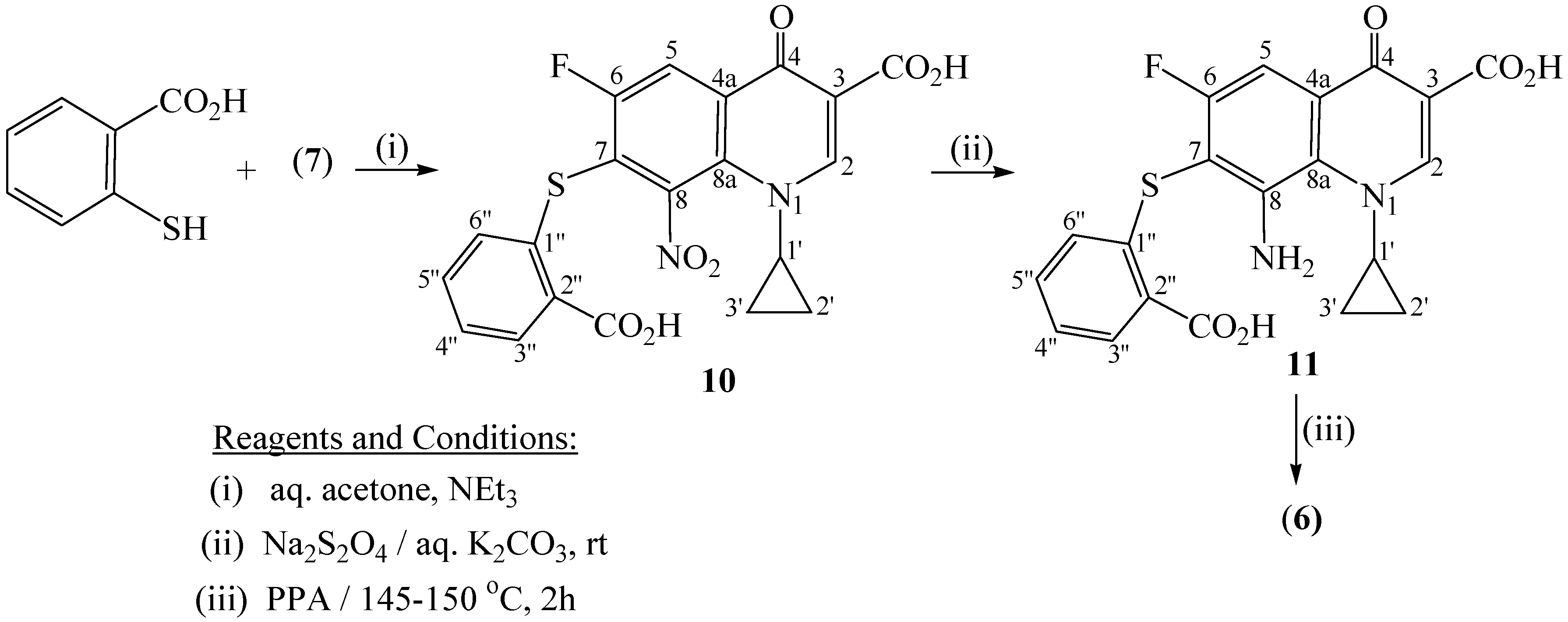
Experimental
General
7-Chloro-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid(7)
7-[(2-Carboxyethyl)thio]-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8)
8-Amino-7-[(2-carboxyethyl)thio]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (9)
11-Cyclopropyl-6-fluoro-2,8-dioxo-1,2,3,4,8,11-hexahydro[1,4]thiazepino[2,3-h]quinoline-9-carboxylic acid (3)
7-[(2-Carboxyphenyl)thio]-1-cyclopropyl -6-fluoro-8-nitro-4-oxo-1,4-dihydroquinolin-3-carboxylic acid (10)
8-Amino-7-[(2-carboxyphenyl)thio]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (11)
1-cyclopropyl-6-fluoro-4,12-dioxo-1,4,12,13-tetrahydroquino[7,8-b]benzothiazepine-3-carboxylic acid (6)
Acknowledgements
References and Notes
- Part III: Abu Shuheil, M. Y.; Hassuneh, M. R.; Al-Hiari, Y. M.; Qaisi, A. M.; El-Abadelah, M. Heterocycles [h]-Fused onto 4-Oxoquinoline-3-carboxylic acid. Facile Synthesis and Antitumor Activity of Model Heterocycles [a]-Fused onto Pyrido[2,3-f] quinoxaline-3-carboxylic Acids. 2007, 71. in press. [Google Scholar] .
- Wise, R.; Andrews, J. M.; Edwards, L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob. Agents Chemother 1983, 23, 559–564. [Google Scholar] Felmingham, D.; O'Hare, M. D.; Robbins, M. J.; Wall, R. A.; Williams, A. H.; Cremer, A. W.; Ridgeway, G. L.; Gruneberg, R. N. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs under experimental and clinical research. Drugs Exp. Clin. Res. 1985, 11, 317–329. [Google Scholar] Maurer, F.; Grohe, K. 2,4-Dichloro-5-fluorobenzoic acid. Ger. Offen. 1986, (Chem. Abstr. 1986, 105, 97158e). 3,435, 392. [Google Scholar] Petersen, U.; Bartel, S.; Bremm, K. D.; Himmler, T.; Krebs, A.; Schenke, T. The synthesis and biological properties of 6-fluoroquinolonecarboxylic acids. Bull. Soc. Chim. Belg. 1996, 105, 683–699. [Google Scholar]
- See for example: Okada, T.; Ezumi, K.; Yamakawa, M.; Sato, H.; Tsuji, T.; Tsushima, T.; Motokawa, K.; Komatsu, Y. Quantitative structure-activity relationships of antibacterial agents, 7-heterocyclic amine substituted 1-cyclopropyl-6,8-difluoro-4-oxoquinoline-3-carboxylic acids. Chem. Pharm. Bul. Jpn. 1993, 41, 126–131. [Google Scholar] ; (b)Grohe, K. in Quinolone Antibacterials. Springer-Verlag: Berlin, Heidelberg, 1998; 13–62. [Google Scholar] ; Li, Q.; Mitscher, L. A.; Shen, L. L. The 2-pyridone antibacterial agents: Bacterial topoisomerase inhibitors. Med. Res. Rev. 2000, 20, 231–293. [Google Scholar] ; Zhanel, G. G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A. S.; Embil, J.; Smith, H.; Hoban, D. A critical review of the fluoroquinolones: Focus on respiratory tract infections. J. Drugs 2002, 62, 13–59. [Google Scholar] ; Da Silva, A. D.; De Almeida, M. V.; De Souza, M. V. N.; Couri, M. R. C. Biological activity and synthetic metodologies for the preparation of fluoroquinolones, a class of potent antibacterial agents. Curr. Med. Chem. 2003, 10, 21–39. [Google Scholar] ; (f)Daneshtalab, M. Topics in Heterocyclic Chemistry. Volume 2, Heterocyclic Antitumor Antibiotics, Springer-Verlag: Berlin / Heidelberg, 2005; 153–173. [Google Scholar] ; Mistcher, L. A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar] .
- Elsea, H. S.; McGuirk, P. R.; Gootz, T. D.; Moynihan, M.; Osheroff, N. Drug features that contribute to the activity of quinolones against mammalian topoisomerase II and cultured cells: correlation between enhancement of enzyme-mediated DNA cleavage in vitro and cytotoxic potential. Antimicrob. Agents Chemother. 1993, 37, 2179–2186. [Google Scholar] Spitzner, J. R.; Chung, I. K.; Gootz, T. D.; McGuirk, P. R.; Muller, M. T. Analysis of eukaryotic topoisomerase II cleavage sites in the presence of the quinolone CP-115,953 reveals drug-dependent and -independent recognition elements. Mol. Pharmacol. 1995, 48, 238–249. [Google Scholar] Riesbeck, K.; Forsgren, A. CP-115,953 Stimulates cytokine production by lymphocytes. Antimicrob. Agents Chemother 1995, 39, 476–483. [Google Scholar] Bromberg, K. D.; Burgin, A. B.; Osheroff, N. Quinolone action against human topoisomerase II : Stimulation of enzyme-mediated double-stranded DNA cleavage. Biochemistry 2003, 42, 3393–3398. [Google Scholar]
- Yoshinari, T.; Mano, E.; Arakawa, H.; Kurama, M.; Iguchi, T.; Nakagawa, S.; Tanaka, N.; Okura, A. Stereo (C7)-dependent topoisomerase II inhibition and tumor growth suppression by a new quinolone, BO-2367. Jpn. J. Cancer Res. 1993, 84, 800–806. [Google Scholar] Okura, A.; Yoshinari, T.; Arakawa, H.; Nakagawa, S.; Mano, E.; Ushijima, R. Preparation of fluoropyridonecarboxylic acid derivatives as antitumor and antibacterial agents. PCT Int. Appl WO 9212146, 1992. [Chem. Abstr. 1993, 118, 147465]. [Google Scholar]
- Arakawa, H.; Mano, E.; Hakoda, N.; Yoshinari, T.; Nakagawa, S.; Okura, A. Potent antitumor activity of quinolone compounds with an unsaturated aminoazabicyclo group at the C-7 position of the quinolone ring. Anti-cancer Drug Des. 1996, 11, 221–229. [Google Scholar] Elsea, S. H.; Westergaard, M.; Burden, D. A.; Lomenick, J.-P.; Osheroff, N. Quinolones share a common interaction domain on topoisomerase II with other DNA. Biochemistry 1997, 36, 2919–2924. [Google Scholar] Kamat, A. M.; DeHaven, J. I.; Lamm, D. L. Quinolone antibiotics: A potential adjunct to intravesical chemotherapy for bladder cancer. Urology 1999, 54, 56–61. [Google Scholar] Nishijima, C.; Kawada, K.; Ohara, K.; Shinomiya, T.; Fukuda, S.; Nakamura, A.; Sawai, T.; Ikekita, M. Novel effects of quinolones to induce apoptosis in a hepatocellular carcinoma cell line, HepG2. Res. Comm. Biochem. Cell Mol. Biol. 2002, 6, 21–38. [Google Scholar] Huang, D.; Okada, K.; Komori, C.; Itoi, E.; Suzuki, T. Enhanced antitumor activity of ultrasonic irradiation in the presence of new quinolone antibiotics in vitro. Cancer Sci. 2004, 95, 845–849. [Google Scholar]
- This drug, namely (+)-cis-3-acetoxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxy-phenyl)-1,5-benzothiazepin-4(5H)-one, was developed and introduced in Japan as a cardio-vascular agent for the treatment of angina pectoris. The drug dilates peripheral arteries and arterioles; it also increases myocardial oxygen supply by relieving coronary artery spasm and reduces myocardial oxygen demand by decreasing heart rate and reducing overload.
- Krapcho, J.; Spitzmiller, E. R.; Turk, C. F. Substituted 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones and 3,4-dihydro-2-phenyl-(2H)-1,6-benzothiazocin-5(6H)-ones. J. Med. Chem. 1963, 6, 544–546. [Google Scholar] Zagorevskii, V. A.; Dudykina, N. V. Ring expansion in reduction of oximes. Zhur. Obshch. Khim. 1963, 33, 322–323. [Google Scholar] Levai, A.; Pazicha, G. Oxazepines and thiazepines. New procedures for the preparation of 2,3-dihydro-1,5-benzothiazepine-4(5H)-ones substituted in position 2. Synth. Commun. 1985, 15, 623–632. [Google Scholar] Ambrogi, V.; Grandolini, G. A convenient one pot synthesis of 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones. Synthesis 1987, 724–726. [Google Scholar] Mais, F. J.; Fiege, H. Preparation of 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones as cocatalysts for Friedel-Craft’s chlorination of alkylbenzenes. Ger. Offen. DE 3, 800,386, 1989. [Google Scholar] Sharma, A. K.; Singh, G.; Yadav, A. K.; Prakash, L. Improved method for the synthesis of new 1,5-benzothiazepine derivatives as analogues of anticancer drugs. Molecules 1989. [Google Scholar]
- Glaser, R.; Sklarz, B. Stereochemistry and conformation in solution of diltiazem hydrochloride, a 1,5-benzothiazepine coronary vasodilator. J. Chem. Soc., Perkin Trans. 2 1989. [Google Scholar] Shibata, S. S.; Satake, N.; Hester, R. K.; Mochizuki, S.; Kosakai, K.; Ueyama, N.; Wakabayashi, S. Characterization of the vasoinhibitory actions of KT2-230, a new benzothiazepine vasodilator derivative on isolated rabbit vascular smooth muscles: An alpha-adrenergic- and serotonergic-receptor antagonist. Gen. Pharmacol. Vasc. Syst. 1991, 22, 443–448. [Google Scholar]
- Pitt, B. Diversity of calcium antagonists. Clin. Ther. 1997, 19, 3–17. [Google Scholar] Smith, D. H. G.; Neutel, J. M.; Weber, M. Comparisons of the effects of different long-acting delivery systems on the pharmacokinetics and pharmacodynamics of diltiazem. Am. J. Hypertens. 1999, 12, 1030–1037. [Google Scholar] Hagiwara, M.; Adachi-Akahane, S.; Nagao, T. High-affinity binding of [3H]DTZ323 to the diltiazem-binding site of L-type Ca2+ channels. Eur. J. Pharmacol. 2003, 466, 63–71. [Google Scholar] Hart, J.; Wilkinson, M. F.; Kelly, M. E. M.; Barnes, S. Inhibitory action of diltiazem on voltage-gated calcium channels in cone photoreceptors. Exp. Eye Res. 2003, 76, 597–604. [Google Scholar]
- Krapcho, J.; Turk, C. F.; Piala, J. Syntheses and pharmacological activity of compounds related to the antidepressant,5-(2-dimethylaminoethyl)-2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-one (thiaze-sim). III. J. Med. Chem. 1968, 11, 361–364. [Google Scholar] [CrossRef]
- Sarro, G. D.; Chimirri, A.; Sarro, A. D.; Gitto, R.; Grasso, S.; Zappala, M. 5H-[1,2,4]Oxadiazolo[5,4-d][1,5]benzothiazepines as anticonvulsant agents in DBA/2 mice. Eur. J. Med. Chem. 1995, 30, 925–929. [Google Scholar]
- Singh, G.; Kumar, N.; Yadav, A. K.; Mishra, A. K. Syntheses of some new 1,5-benzothiazepine derivatives and their ribofuranosides as antimicrobial agents. Heteroat. Chem. 2002, 13, 620–625. [Google Scholar] [CrossRef]
- Kwak, B.-sung; Chung, K.-nam; Koh, K.-ho; Hwang, H.-jun. Method of preparing 10H-dibenzo[b,f]]1,4]thiazepin-11-one. PCT Int. Appl. WO 047722, 2004. [Google Scholar]
- Nicol, R. H.; Slater, M. J.; Hodgson, S. T. Preparation of dibenzothiazepinethiones as antiviral agents. PCT Int. Appl. WO 19607, 1992. [Google Scholar] Nicol, R. H.; Slater, M. J.; Hodgson, S. T. Preparation of 10,11-dihydrobenzo[b,f][1,4]thiazepin-11-ones as virucides. PCT Int. Appl. WO 19277, 1992. [Google Scholar]
- Hargrave, K. D.; Schmidt, G.; Engel, W.; Schromm, K. Preparation of dibenz[b,f][1,4]oxazepin (and thiazepin)-11(10H)-ones and-thiones for prevention and treatment of AIDS. Can. Pat. Appl. 2024040, 1991. [Google Scholar]
- Belanger, P. C.; Rokach, J.; Scheigetz, J. Preparation of 10,11-dihydrodibenzo[b,f][1,4]thiazepines as leukotriene antagonists. U.S. Pat. 4728735, 1988. [Google Scholar]
- Holkar, A. G.; Pise, A. Processes for the one-pot preparation of thiazepines and their pharmaceutical compositions. C. PCT Int. Appl. WO 020011, 2007. [Google Scholar] Tarur, V. R.; Sathe, D. G.; Naidu, A. V.; Aher, U. P.; Patil, S. S. A Process for the preparation of quetiapine hemifumarate. PCT Int. Appl. WO 004234, 2007. [Google Scholar] Buenger, G. S.; Alexander, A. Preparation of 11-{4-[2-(2-hydroxyethoxy)ethyl]piperazinyl}dibenzo[b,f][1,4]thiazepine (quetiapine) and its fumarate salt via chlorination of dibenzo[b,f][l,4]thiazepine-11(10H)one with phosphorus oxychloride. PCT Int. Appl. WO 135544, 2006. [Google Scholar] Bosch, L. J.; Burgarolas, M. M. C.; Chamorro, G. I. Process for preparing quetiapine and quetiapine fumarate by treatment of dibenzo[b,f][1,4]thiazepin-11(10H)-one with phosphorus oxychloride and then with 2-(2-piperazin-1-ylethoxy)ethanol. PCT Int. Appl. WO 117700, 2006. [Google Scholar] Harada, K.; Nishino, S.; Yoshii, K. Preparation of dibenzothiazepines and their intermediates. Jpn. Kokai Tokkyo Koho. JP 11199574, 1999. [Google Scholar]
- Davis, P. C.; Goldstein, J. M.; Grimm, S. W.; Winter, H. R.; Suckow, R. F. Method using 11-piperazin-1-yldibenzo[b,f][1,4]thiazepine for treating schizophrenia and other disorders. U.S. Pat. Appl. 217366, 2006. [Google Scholar]
- Davis, P. C.; Goldstein, J. M.; Grimm, S. W.; Suckow, R. F.; Winter, H. R. Use of 11-piperazin-1-yldibenzo[b,f][1,4]thiazepine or a pharmaceutically acceptable salt thereof to treat substance-related and other disorders, and oral pharmaceutical compositions. PCT Int. Appl. WO 073360, 2006. [Google Scholar]
- Li, R.; Farmer, P. S.; Wang, J.; Boyd, R. J.; Quilliam, M. A.; Walter, J. A.; Howlett, S. E. Molecular geometries of dibenzothiazepinone and dibenzoxazepinone calcium antagonist. Drug Design and Discovery 1995. [Google Scholar] Li, R.; Farmer, P. S.; Quillam, M. A.; Howlett, S. E. Dibenzothiazepinones as potential calcium channel antagonists. Drug Design and Discovery. 1993. [Google Scholar]
- Warawa, E. J.; Migler, B. M. Preparation of 11-{4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl} dibenzo[b,f][1,4]thiazepine as a neuroleptic and antipsychotic. Eur. Pat. Appl. EP 240228, 1987. [Google Scholar]
- Barker, A. C.; Copeland, R. J. Process for the preparation of a piperazinodibenzothiazepine with antidopaminergic activity. Eur. Pat. Appl. 1988. [Google Scholar]
- Sanchez, J. P.; Domagala, J. M.; Hagen, S. E.; Heifetz, C. L.; Hutt, M. P.; Nichols, J. B.; Trehan, A. K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J. Med. Chem. 1988, 31, 983–991. [Google Scholar]
- Grohe, K.; Heitzer, H. Cycloaracylation of enamines. I. Synthesis of 4-quinolone-3-carboxylic acids. Liebigs Ann. Chem. 1987, 29–37. [Google Scholar] Petersen, U.; Grohe, K.; Schenke, T.; Hagemann, H.; Zeiler, H. J.; Metzger, K. G. Preparation of 7-(azabicycloalkyl)-3-quinolinecarboxylates and -3-naphthyridinecarboxylates as bactericides and feed additives. Ger. Offen. 3 601 567, 1987. [Google Scholar]
- Pulla, R. M.; Venkaiah, C. N. An improved process for the preparation of quinolone derivatives, e.g. ciprofloxacin. PCT Int. Appl. WO 085 692, 2001. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Al-Huniti, M.H.; El-Abadelah, M.M.; Zahra, J.A.; Sabri, S.S.; Ingendoh, A. Heterocycles [h]Fused onto 4-Oxoquinoline-3-Carboxylic Acid, Part IV. Convenient Synthesis of Substituted Hexahydro [1,4]Thiazepino[2,3-h]quinoline-9-carboxylic Acid and Its Tetrahydroquino[7,8-b]benzothiazepine Homolog. Molecules 2007, 12, 1558-1568. https://doi.org/10.3390/12081558
Al-Huniti MH, El-Abadelah MM, Zahra JA, Sabri SS, Ingendoh A. Heterocycles [h]Fused onto 4-Oxoquinoline-3-Carboxylic Acid, Part IV. Convenient Synthesis of Substituted Hexahydro [1,4]Thiazepino[2,3-h]quinoline-9-carboxylic Acid and Its Tetrahydroquino[7,8-b]benzothiazepine Homolog. Molecules. 2007; 12(8):1558-1568. https://doi.org/10.3390/12081558
Chicago/Turabian StyleAl-Huniti, Mohammed H., Mustafa M. El-Abadelah, Jalal A. Zahra, Salim S. Sabri, and Arnd Ingendoh. 2007. "Heterocycles [h]Fused onto 4-Oxoquinoline-3-Carboxylic Acid, Part IV. Convenient Synthesis of Substituted Hexahydro [1,4]Thiazepino[2,3-h]quinoline-9-carboxylic Acid and Its Tetrahydroquino[7,8-b]benzothiazepine Homolog" Molecules 12, no. 8: 1558-1568. https://doi.org/10.3390/12081558



