Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity
Abstract
:Introduction
Results and Discussion
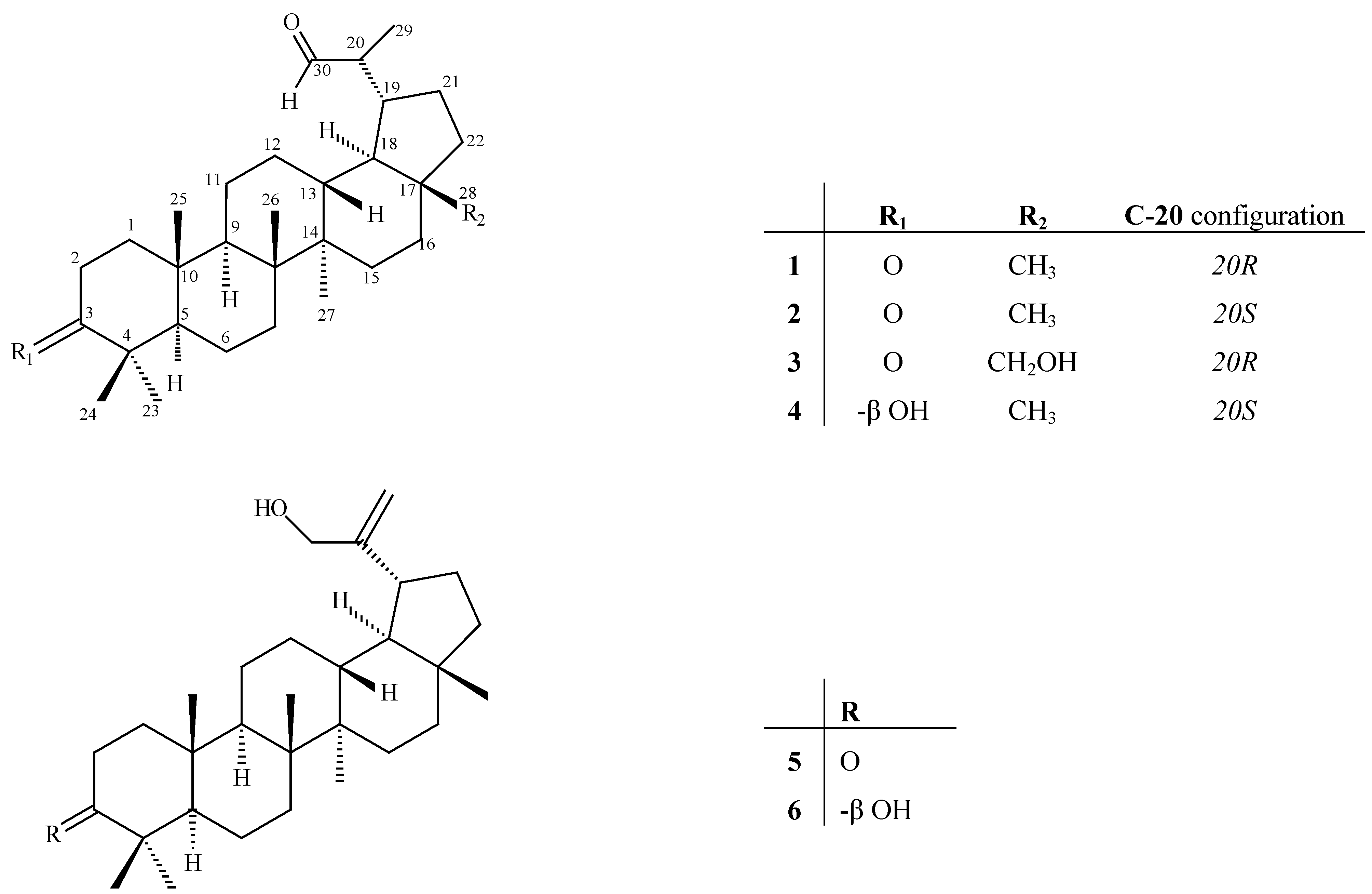
| # | 1 (δH) | 1 (δC) | 2 (δH) | 2 (δC) | 3 (δH) | 3 (δC) | 4 (δH) | 4 (δC) |
|---|---|---|---|---|---|---|---|---|
| 1 | α,1.40 (m) β,1.91 (m) | 39.4 | α, 1.90 (ddd, 4.4, 7.6, 13.2) β, 1.38 (m) | 39.5 | α,1.39 (m) β,1.91 (m) | 39.5 | α, 0.90(m) β, 1.63 (m) | 38.7 |
| 2 | α, 2.40 (ddd, 4.7, 7.8, 15.7) β, 2.47 (ddd, 7.5, 9.6, 15.7) | 34.0 | α, 2.40 (m), β, 2.47 (ddd, 7.3, 9.7, 15.9) | 34.1 | α, 2.42 (ddd, 4.4, 7.9, 15.8) β, 2.46 (ddd, 7.5, 9.8, 15.8) | 34.1 | α, 1.58 (m) β, 1.63 (m) | 27.4 |
| 3 | 218.5 | 217.8 | 218.0 | 3.18 (dd, 4.4, 10.6) | 79.0 | |||
| 4 | 47.3 | 47.3 | 47.3 | 38.9 | ||||
| 5 | 1.33 (m) | 54.7 | 1.32 (m) | 54.8 | 1.34 (m) | 54.7 | 0.67 (dd, 2.72, 9.20) | 55.2 |
| 6 | α,1.44 (m) β,1.56 (m) | 19.6 | 2H, 1.41-1.53 (m) | 19.6 | 2Η ,1.48 (m) | 19.7 | α, 1.38 (m) β, 1.52 (m) | 18.3 |
| 7 | 2H, 1.44 (m) | 33.4 | 2H, 1.45 (m) | 33.6 | 2Η , 1.44 (m) | 33.5 | 2H, 1.37 (m) | 34.3 |
| 8 | 40.6 | 40.8 | 40.9 | 40.8 | ||||
| 9 | 1.38 (m) | 49.2 | 1.38 (m) | 49.3 | 1.40 (m) | 49.3 | 1.28 (m) | 50.0 |
| 10 | 36.7 | 36.8 | 36.8 | 37.1 | ||||
| 11 | α, 1.55 (m) β, 1.40 (m) | 21.3 | α, 1.06 (m) β, 1.52 (m) | 21.2 | α, 1.51 (m) β, 1.35 (m) | 21.3 | α, 1.47 (m) β, 128 (m) | 20.7 |
| 12 | α, 1.38 (m) β, 1.56 (m) | 27.4 | α, 1.35 (m) β, 1.65 (m) | 26.5 | α, 1.34 (m) β, 1.55 (m) | 27.6 | α, 1.65 (m) β, 1.38 (m) | 26.5 |
| 13 | 1.74 (m) | 37.8 | 1.79 (ddd, 4.1, 11.7, 11.7) | 37.8 | 1.70 (m) | 37.0 | 1.77 (ddd 4.4, 11.6 , 11.6) | 37.7 |
| 14 | 42.9 | 43.1 | 42.9 | 42.8 | ||||
| 15 | α, 1.05 (m) β, 1.69 (m) | 27.1 | α, 1.03 (m) β, 1.69 (m) | 27.2 | α, 1.07 (m) β, 1.72 (m) | 26.8 | α, 1.03 (m) β, 1.70 (m) | 27.2 |
| 16 | 2H, 1.47 (m) | 35.1 | a, 1.46 (m) b, 1.53 (m) | 35.2 | α, 1.19 (m) β, 1.92 (m) | 29.1 | α, 1.48 (m) β, 1.59 (m) | 35.3 |
| 17 | 43.0 | 42.9 | 47.8 | 43.0 | ||||
| 18 | 1.43 (m) | 48.9 | 1.26 (m) | 47.0 | 1.64 (m) | 49.3 | 1.24 (m) | 47.1 |
| 19 | 1.89 (m) | 42.6 | 2.35 (m) | 37.3 | 1.91 (m) | 42.7 | 2.35 (m) | 37.4 |
| 20 | 2.60 (m) | 48.9 | 2.63 (dq, 2.9, 6.8) | 49.7 | 2.57 (m) | 49.0 | 2.64 (dq, 3.1, 6.8) | 49.7 |
| 21 | α, 1.51 (m) β, 1.88 (m) | 25.0 | α, 1.15 (m) β, 1.68 (m) | 23.6 | α, 1.55 (m) β, 1.92 (m) | 24.9 | α, 1.15 (m) β, 1.68 (m) | 23.6 |
| 22 | α, 1.36 (m) β, 1.47 (m) | 39.9 | α, 1.39 (m) β, 1.45 (m) | 40.4 | α, 0.98(m) β,1.92 (m) | 33.7 | α, 1.08 (m) β, 1.38 (m) | 40.4 |
| 23 | 1.06 (s) | 26.6 | 1.06 (s) | 26.6 | 1.06 (s) | 26.7 | 0.96 (s) | 28.0 |
| 24 | 1.01 (s) | 21.0 | 1.01 (s) | 21.1 | 1.01 (s) | 21.1 | 0.75 (s) | 15.4 |
| 25 | 0.91 (s) | 15.7 | 0.93 (s) | 15.9 | 0.91 (s) | 16.0 | 0.82 (s) | 16.0 |
| 26 | 1.06 (s) | 15.7 | 1.07 (s) | 15.8 | 1.06 (s) | 15.8 | 1.03 (s) | 15.9 |
| 27 | 0.92 (s) | 14.2 | 0.93 (s) | 14.3 | 0.94 (s) | 14.5 | 0.92 (s) | 14.3 |
| 28 | 0.75 (s) | 17.5 | 0.78 (s) | 17.9 | α, 3.26 (d, 10.9) β, 3.76 (d, 10.9) | 60.1 | 0.78 (s) | 17.9 |
| 29 | 1.07 (d, 7.2) | 14.4 | 1.01 (d, 6.8) | 7.4 | 1.09 (d, 7.20) | 14.5 | 1.01 (d, 6.8) | 7.4 |
| 30 | 9.84 (d, 2.0) | 207.1 | 9.60 (s) | 205.1 | 9.83 (d, 2.0) | 206.7 | 9.60 (s) | 205.1 |
Experimental
General
Plant Material
Extraction and Isolation
Evaluation of Cytotoxicity
Acknowledgments
References
- Harden, G.J. (Ed.) Flora of New South Wales; Vol. 2, New South Wales Univ. Press: NSW Australia, 1991.
- Doran, J.C.; Turnbull, J.W.; Boland, D.J.; Gunn, B.V. Handbook on seeds of dry zone Acacias; FAO: Rome, 1983. [Google Scholar]
- Ross, J.H. A conspectus of the African Acacia species. Mem. Bot. Soc. South Africa 1979, 44, 1–155. [Google Scholar]
- Hooker, J.D. The flora of British India; Vol. 3, L. Reeve and Co: London, 1879. [Google Scholar]
- Brandis, D. Indian trees; Archibald Constable & Co. Ltd.: London, 1906. [Google Scholar]
- Kokwaro, O. Medicinal Plant of East Africa; East African Literature Bureau Kampala: Nairobi, Dar es Salaam, 1976. [Google Scholar]
- Johns, S.; Lumberton, J.; Sioumis, A. Alkaloids of the Australian Leguminosae. Aust. J. Chem. 1966, 19, 893–896. [Google Scholar] [CrossRef]
- Imperato, F. A chalcone glycoside from Acacia dealbata. Phytochemistry 1982, 21, 480–481. [Google Scholar] [CrossRef]
- Foster, P.; Jefferies, P. Labdane diterpenes from an Acacia species. Phytochemistry 1985, 24, 2991–2993. [Google Scholar] [CrossRef]
- Heerden, F.; Brandt, E.; Ferreira, D.; Roux, D. Metabolites from the purple heartwoods of the Mimosoidae. Part 4. Acacia fasciculifera F. Muell ex. Benth: fasciculiferin, fasciculiferol, and the synthesis of 7-aryl-and 7-flavanyl-peltogynoids. J. Chem. Soc. Perkin Trans. I 1981, 2483–2490. [Google Scholar]
- Anjaneyulu, A.; Bapuji, M.; Ramachandra, L.; Row Sree, A. Structure of acacigenin-B, a novel triterpene ester isolated from Acacia concinna. Phytochemistry 1979, 18, 463–465. [Google Scholar] [CrossRef]
- Pereira, F.B.M.; Domingues, F.M.J.; Silva, A.M.S. Triterpenes from Acacia dealbata. Nat. Prod. Lett. 1996, 8, 97–103. [Google Scholar] [CrossRef]
- Kulshreshtha, D.K. Three new oxygenated triterpenoids of the lupane series from Gymnosporia wallichiana. Phytochemistry 1977, 16, 1783–1785. [Google Scholar] [CrossRef]
- Corbett, R.E.; Cong, A.N.T.; Holland, P.T.; Wilkins, A.L. Extractives from Pseudocyphellaria rubella. Aust. J. Chem. 1987, 40, 461–468. [Google Scholar] [CrossRef]
- Christodoulopoulou, L.; Tsoukatou, M.; Tziveleka, L.A.; Vagias, C.; Petrakis, P.V.; Roussis, V. Piperidinyl amides with insecticidal activity from the maritime plant Otanthus maritimus. J. Agr. Food Chem. 2005, 53, 1435–1439. [Google Scholar]
- Kladi, M.; Xenaki, H.; Vagias, C.; Papazafiri, P.; Roussis, V. New cytotoxic sesquiterpenes from the red algae Laurencia obtusa and Laurencia microcladia. Tetrahedron 2006, 62, 182–189. [Google Scholar]
- Mutai, C.; Abatis, D.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Cytotoxic lupane-type triterpenoids from Acacia mellifera. Phytochemistry 2004, 65, 1159–1164. [Google Scholar] [CrossRef]
- Tinto, W.F.; Blair, L.C.; Alli, A. Lupane triterpenoids of Salacia cordata. J. Nat. Prod. 1992, 55, 395–1992. [Google Scholar] [CrossRef]
- Abdel-Mogib, M. A lupane triterpenoid from Maerua oblongifolia. Phytochemistry 1999, 51, 445–448. [Google Scholar]
- Burns, D.; Reynolds, W.F.; Buchanan, G.; Reese, P.B.; Enriquez, R.G. Assignment of 1H and 13C spectra and investigation of hindered side-chain rotation in lupeol derivatives. Magn. Reson. Chem. 2000, 38, 488–493. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, H.S.; Yook, C.S.; Nohara, T. A new lupane glycoside from the leaves of Acanthopanax koreanum. Chem. Pharm. Bull. 2005, 53, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Kuo, Y.H. New peroxy triterpenes from the aerial roots of Ficus microcarpa. J. Nat. Prod. 2001, 64, 436–439. [Google Scholar]
- Corbett, R.E.; Cong, A.N.T.; Wilkins, A.L.; Thomson, R.A. Lichens and fungi. Part 17. The synthesis and absolute configuration at C-20 of the (R) -and (S)- epimers of some 29-substituted lupane derivatives and of some 30-norlupan-20-ol derivatives and the crystal structure of (20R)-3β-acetoxylupan-29-ol. J. Chem. Soc. Perkin Trans. I 1985, 2051–2056. [Google Scholar]
- Drewes, S.E.; Mashimbye, M.J. Flavanoids and triterpenoids from Cassine papillosa and the absolute configuration of 11,11-dimethyl-1,3,8,10-tetra-hydroxy-9-methoxypeltogynan. Phytochemistry 1993, 32, 1041–1044. [Google Scholar] [CrossRef]
- Betancor, C.; Freire, R.; Gonzalez, A.G.; Salazar, J.A.; Pascard, C.; Prange, T. Three triterpenes and other terpenoids from Catha cassinoides. Phytochemistry 1980, 19, 1989–1993. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I. Identification of Lichens Substances; Springer Verlag: Berlin, 1996. [Google Scholar]
- De Carvalho, M.G.; De Carvalho, G.J.A.; Braz-Filho, R. Chemical constituents from Ouratea floribunda: Complete 1H and 13C NMR. Assignments of atranorin and its new acetyl derivative. J. Braz. Chem. Soc. 2000, 11, 143–147. [Google Scholar]
- Masan, C.; Woitke, H.D.; Hiller, K.; Franke, P. Isolation of beta-sitosterol-O-D-glucoside from Astrantia major L. 31. The contents of some Saniculoideae. Pharmazie 1978, 33, 382. [Google Scholar]
- Grindley, D.N. Investigation of the seed oils of some Sudan Mimosaceae. J. Soc. Chem. Ind. 1945, 64, 152. [Google Scholar]
- Ogura, M.; Cordell, G.A.; Farnsworth, N.R. Potential anticancer agents. IV. Constituents of Jacaranda causana Pittier (Bignoninaceae). Lloydia 1977, 40, 157–168. [Google Scholar]
- Sandberg, F.; Duchevska, K.; Khristov, V.; Spasov, S. Spondianthus preussii var. grabber Engler. Pharmacological screening and occurrence of triterpenes. Acta Pharm. Suec. 1987, 24, 253–256. [Google Scholar]
- Ryu, S.Y.; Choi, S.U.; Lee, S.H.; Lee, C.O.; Zaesung, N.; Ahn, J. Antitumor triterpenes from medicinal plants. Arch. Pharmacal Res. 1994, 17, 375–377. [Google Scholar] [CrossRef]
- Hata, K.; Hori, K.; Ogasawara, H.; Takahashi, S. Anti-leukemia activities of lup-28-al-20(29)-en-3-one, a lupane triterpene. Toxicol. Lett. 2003, 143, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roussakis, C.; Gratas, C.; Audoin, A.F.; Le Boterf, J.; Dabouis, G.; Verbist, J.F. Study of in vitro drug sensitivity on a newly established cell line from a primary bronchial epidermoid carcinoma of human origin (NSCLC-N6). Anticancer Res. 1991, 11, 2239–2244. [Google Scholar] [PubMed]
- Mossmann, T. Rapid calorimetric assay cellular growth and survival: application to proliferation and cytotoxicity assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1-6 are available from the corresponding author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mutai, C.; Abatis, D.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity. Molecules 2007, 12, 1035-1044. https://doi.org/10.3390/12051035
Mutai C, Abatis D, Vagias C, Moreau D, Roussakis C, Roussis V. Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity. Molecules. 2007; 12(5):1035-1044. https://doi.org/10.3390/12051035
Chicago/Turabian StyleMutai, Charles, Dennis Abatis, Constantinos Vagias, Dimitri Moreau, Christos Roussakis, and Vassilios Roussis. 2007. "Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity" Molecules 12, no. 5: 1035-1044. https://doi.org/10.3390/12051035
APA StyleMutai, C., Abatis, D., Vagias, C., Moreau, D., Roussakis, C., & Roussis, V. (2007). Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity. Molecules, 12(5), 1035-1044. https://doi.org/10.3390/12051035






