Cytotoxic Triterpenoid Saponins from the Roots of Platycodon grandiflorum
Abstract
:Introduction
Results and Discussion
Structure Elucidation
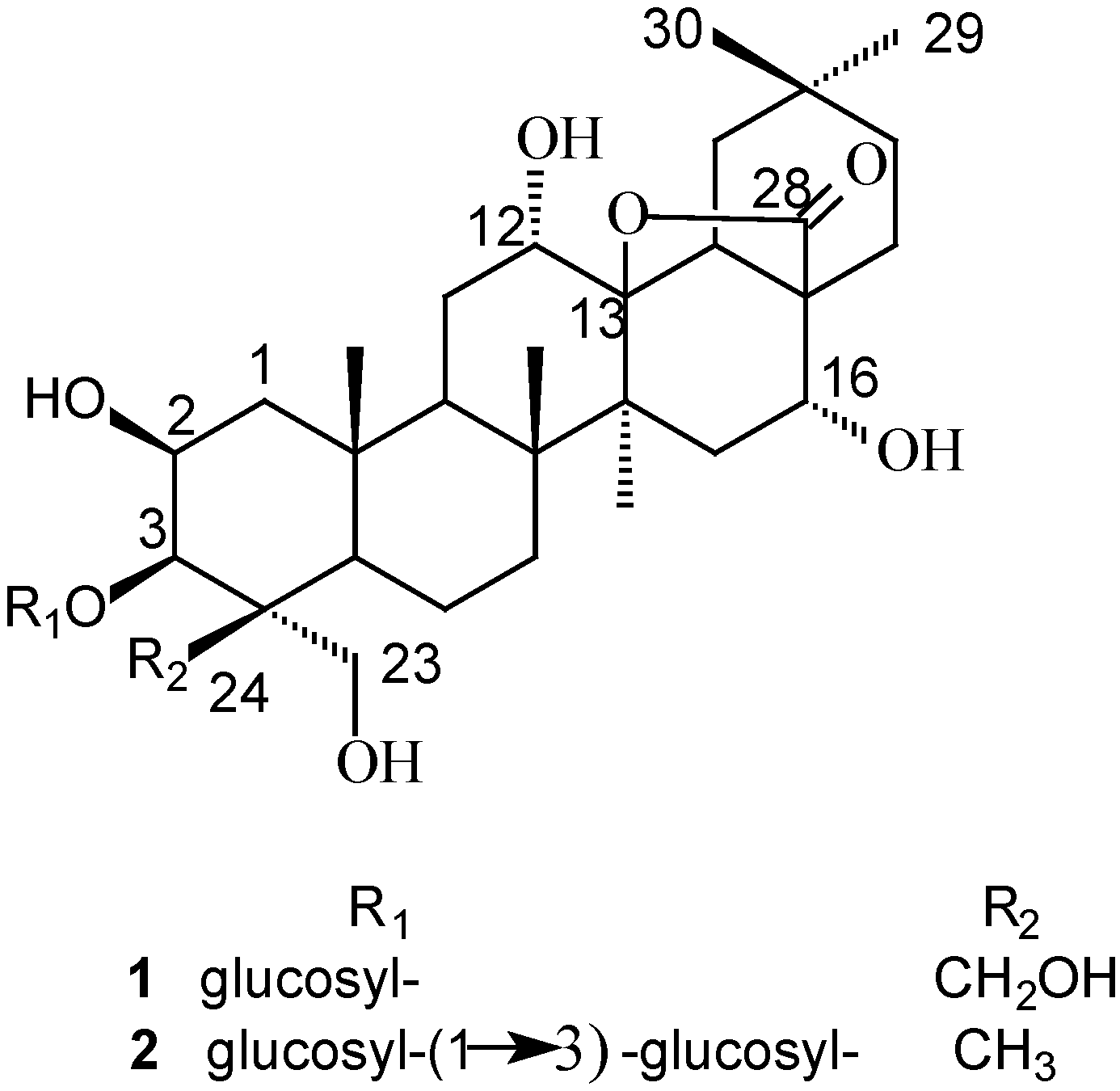
| Position | 1 | 2 | ||
|---|---|---|---|---|
| C | H (J, Hz) | C | H (J Hz) | |
| 1 | 45.8 | 2.48, d, (7.0) | 44.6 | 2.41, d, (7.2) |
| 2.87, d, (7.9) | 2.85, d, (7.6) | |||
| 2 | 69.2 | 4.78, m | 70.8 | 4.78, m |
| 3 | 87.8 | 4.68, m | 82.8 | 4.32, m |
| 4 | 48.4 | 43.2 | ||
| 5 | 46.1 | 0.92, dd, (6.6, 7.0) | 47.9 | 0.83, dd, (6.4, 6.9) |
| 6 | 18.9 | 17.4 | ||
| 7 | 35.3 | 35.0 | ||
| 8 | 42.8 | 42.8 | ||
| 9 | 45.3 | 2.20, dd, (7.2, 7.8) | 45.1 | 2.22, dd, (7.3, 8.0) |
| 10 | 37.5 | 36.7 | ||
| 11 | 30.5 | 1.84, m | 30.2 | 1.87, m |
| 2.37, m | 2.40, m | |||
| 12 | 66.1 | 4.54, m | 66.2 | 4.46, m |
| 13 | 92.8 | 92.6 | ||
| 14 | 43.3 | 43.1 | ||
| 15 | 39.8 | 2.43, m | 39.8 | 2.37, m |
| 16 | 72.7 | 4.51, m | 72.7 | 4.43, m |
| 17 | 49.1 | 49.0 | ||
| 18 | 53.3 | 53.2 | ||
| 19 | 40.8 | 40.7 | ||
| 20 | 32.0 | 31.9 | ||
| 21 | 35.8 | 2.54, dd, (7.0, 7.6) | 35.7 | 2.48, dd, (7.0, 7.5) |
| 22 | 29.0 | 2.07, dd, (7.0, 15.5) | 29.0 | 2.02, dd, (7.0, 15.0) |
| 2.26, dd, (7.6, 15.5) | 2.30, dd, (7.5, 15.0) | |||
| 23 | 63.3 | 4.30, m | 65.0 | 4.10, m |
| 4.86, m | 4.52, m | |||
| 24 | 66.1 | 4.24, m | 14.9 | 1.31, s |
| 4.75, m | ||||
| 25 | 20.2 | 1.52, s | 18.8 | 1.51, s |
| 26 | 18.4 | 1.34, s | 18.5 | 1.30, s |
| 27 | 21.0 | 1.94, s | 21.1 | 1.89, s |
| 28 | 178.1 | 178.1 | ||
| 29 | 33.4 | 1.09, s | 33.4 | 1.01, s |
| 30 | 24.6 | 1.09, s | 24.5 | 1.01, s |
| 1′ | 106.5 | 5.14, d, (7.7) | 105.6 | 5.14, d, (7.0) |
| 2′ | 75.4 | 3.80 | 75.3 | 3.82 |
| 3′ | 78.9 | 4.05 | 88.8 | 3.93 |
| 4′ | 71.8 | 3.96 | 69.7 | 3.97 |
| 5′ | 78.9 | 3.87 | 77.9 | 3.86 |
| 6′ | 62.7 | 4.04, 4.18 | 62.6 | 4.05, 4.19 |
| 1″ | 106.0 | 5.22, d, (6.6) | ||
| 2″ | 75.6 | 3.77 | ||
| 3″ | 78.8 | 4.02 | ||
| 4″ | 71.7 | 3.94 | ||
| 5″ | 78.3 | 3.85 | ||
| 6″ | 62.3 | 3.99, 4.15 | ||
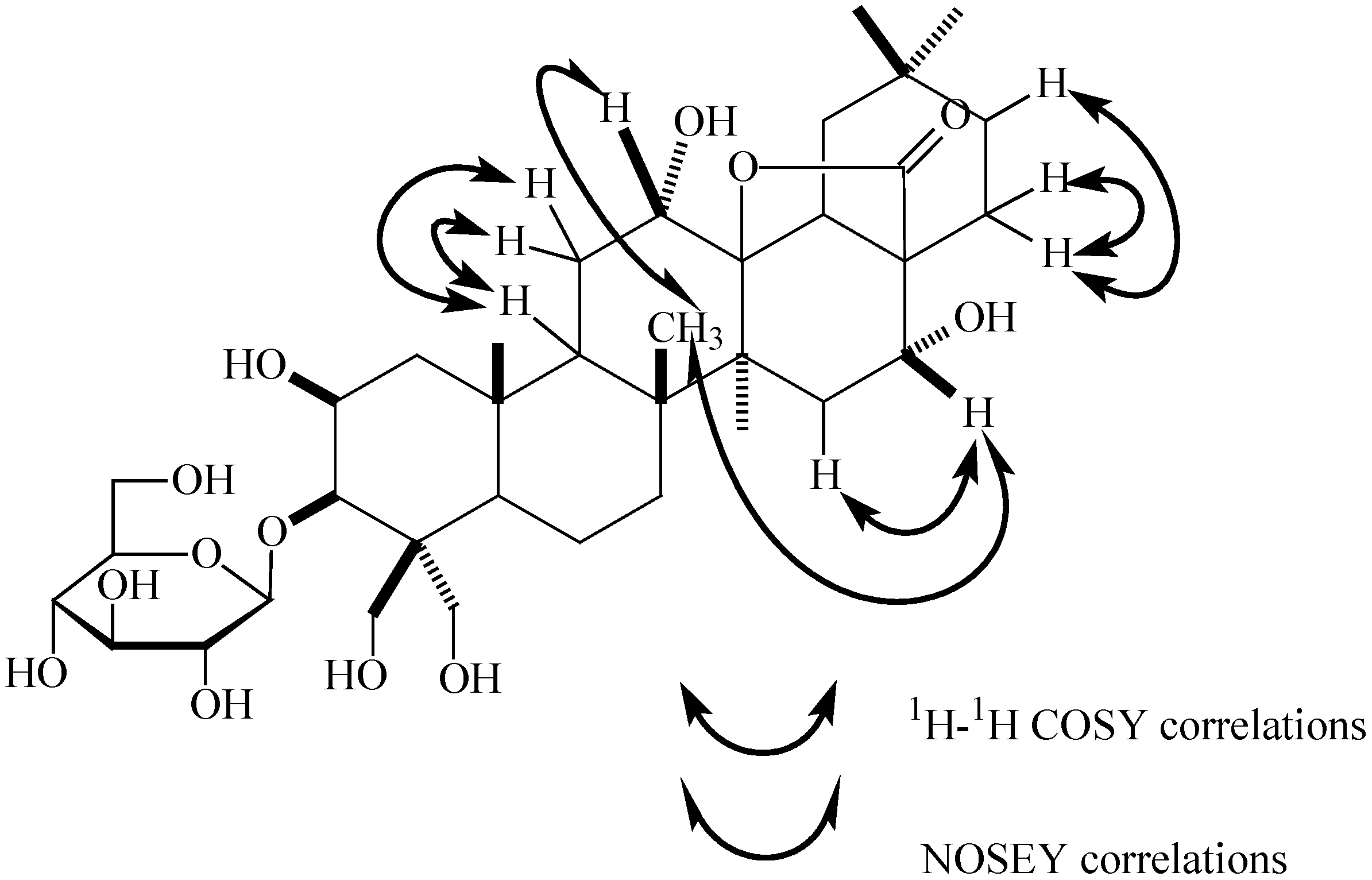
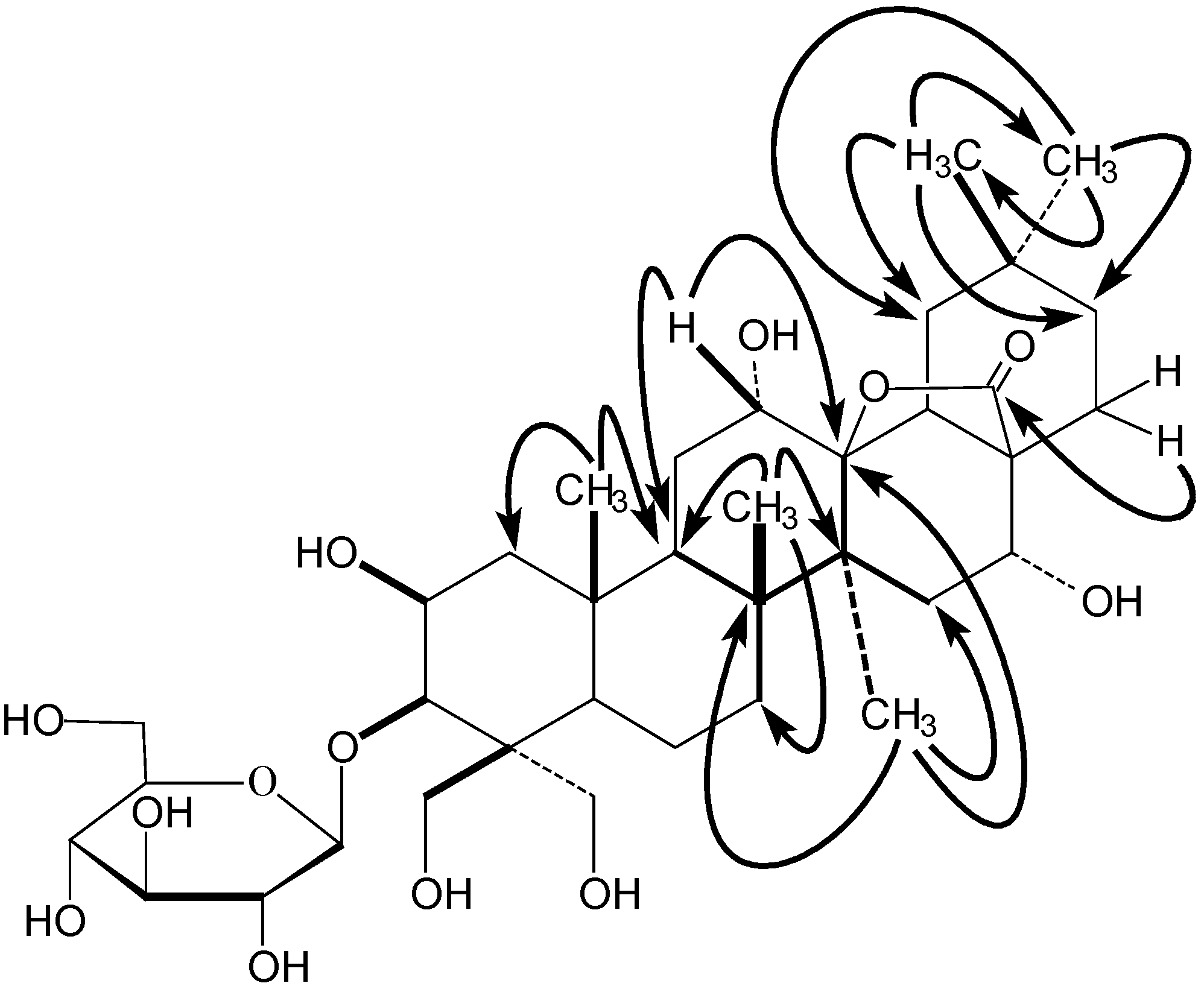
Cytotoxic activity
Conclusions
Experimental
General
Plant Material
Extraction and Isolation
Acid Hydrolysis
Cytotoxicity Assays
Acknowledgments
References
- Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, 2005; Vol. I, p. 196.
- Akiyama, T.; Iitaka, Y.; Tanaka, O. Structure of platicodigenin, a saogenin of Platycodon grandiflorum A. De Candolle. Tetrahedron Lett. 1968, 53, 5577–5580. [Google Scholar] [CrossRef]
- Fu, W.W.; Dou, D.Q.; Shimizu, N.; Takeda, T.; Fu, R.; Pei, Y.H.; Chen, Y.J. Studies on the chemical constituents from the roots of Platycodon grandiflorum. J. Nat. Med. 2006, 60, 68–72. [Google Scholar]
- Fu, W.W.; Hou, W.B.; Dou, D.Q.; Hua, H.M.; Gui, M.H.; Fu, R.; Chen, Y.J.; Pei, Y.H. Saponins of polygalacic acid type from Platycodon grandiflorum. Acta Pharm. Sin. 2006, 41, 358–360. [Google Scholar]
- Fu, W.W.; Shimizu, N.; Dou, D.Q.; Takeda, T.; Fu, R.; Pei, Y.H.; Chen, Y.J. Five new triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006, 54, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Shimizu, N.; Takeda, T.; Dou, D.Q.; Chen, B.H.; Pei, Y.H.; Chen, Y.J. New A-ring lactone triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006, 54, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Qiao, C.F.; Han, Q.B.; Wang, Y.; Ye, W.C.; Xu, H.X. New triterpenoid saponins from the roots of Platycodon grandiflorum. Tetrahedron 2005, 61, 2211–2215. [Google Scholar] [CrossRef]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Structures of Polygalacin-D and -D2, and Their Monoacetates, Saponins isolated from Platycodon grandiflorum A. DC., determined by Carbon-13 Nuclear Magnetic Resonance Spectroscopy. Chem. Pharm. Bull. 1978, 26, 674–677. [Google Scholar]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Structures of platycodin-D3, platyconic acid -A, and their derivatives, saponins isolated from roots of Platycodon grandiflorum A. De Candolle, determined by carbon-13 NMR spectroscopy. Chem. Lett. 1978, 719–722. [Google Scholar]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Saponins from roots of Platycodon grandiflorum. Part 1. Structure of prosapogenins. J. Chem. Soc. Perkin Trans. 1 1981, 1928–1933. [Google Scholar] [CrossRef]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Saponins from roots of Platycodon grandiflorum. Part 2. Isolation and structure of new triterpene glycosides. J. Chem. Soc. Perkin Trans. 1984, 661–668. [Google Scholar]
- Kim, Y.-S.; Kim, J.-S.; Choi, S.-U.; Kim, J.-S.; Lee, H.-S.; Roh, S.-H.; Jeong, Y.-C.; Kim, Y.-K.; Ryu, S.-Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Rada, A.; Shoji, J.; Kasai, R.; Tanaka, O. The Structures of Platcodin A and C, Monoacetylated Saponins of the Roots of Platycodon grandiflorum A. DC. Chem. Pharm. Bull. 1978, 26, 668–670. [Google Scholar] [CrossRef]
- Kubota, T.; Kitatani, H.; Hinoh, H. Structure of platycogenic acids A, B and C Further Trierpenoid Constituents of Platycodon grandiflorum. J. Chem. Soc. D. 1969, 22, 1313–1314. [Google Scholar] [CrossRef]
- Nikaido, T.; Koike, K.; Mitsunaga, K.; Saeki, T. Tirterpenoid saponins from root of Platycodon grandiflorum. Nat. Med. 1998, 52, 54–59. [Google Scholar]
- Nikaido, T.; Koike, K.; Mitsunaga, K.; Saeki, T. Two New Triterpenoid Saponins from Platycodon grandflorum. Chem. Pharm. Bull. 1999, 47, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Kaneiwa, Y.; Shoji, J.; Shibata, S. Studies on the saponins of the Roots of Platycodon grandiflorum A. De Candolle. I. Isolation and the Stricture of Platycodin-D. Chem. Pharm. Bull. 1975, 23, 2965–2972. [Google Scholar]
- Fu, W.W.; Dou, D.Q.; Zhao, C.J.; Shimizu, N.; Pei, Y.P.; Pei, Y.H.; Takeda, T.; Chen, Y.J.; Takeda, T. Triterpenoid saponins from Platycodon grandiflurum. J. Asia Nat. Prod. Res. 2007, 9, 35–40. [Google Scholar] [CrossRef]
- Kim, Y.-P.; Lee, E.-B.; Kim, S.-Y.; Li, D.-W.; Ban, H.-S.; Lim, S.-S.; Shin, K.-H.; Ohuchi, K. Inhibition of Prostaglandin E2 Production by Platycodin D Isolated from the Root of Platycodon grandiflorum. Planta Med. 2001, 67, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Hwang, Y.-P.; Kim, D.-H.; Han, E.-H.; Chung, Y.-C.; Roh, S.-H.; Jeong, H.-G. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci. Biotech. Biochem. 2006, 70, 858–864. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, D.-H.; Kim, H.-G.; Song, G.-Y.; Chung, Y.-C.; Roh, S.-H.; Jeong, H.-G. Inhibition of tumor necrosis factor-alpha-induced expression of adhesion molecules in human endothelial cells by the saponins derived from roots of Platycodon grandiflorum. Toxicol. Appl. Pharmacol. 2006, 210, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Sasada, M.; Yamamoto, K.; Nishiyama, H.; Nishimura, T.; Nakamura, T.; Uchino, H. Immune Pharmacological Studies on Platycodi Radii (I). Effect on the Phagocytosis in Mouse. Shoyakugaku Zasshi 1986, 40, 367–374. [Google Scholar]
- Lee, K.-J.; Kim, J.-Y.; Choi, J.-H.; Kim, H.-G.; Chung, Y.-C.; Roh, S.-H.; Jeong, H.-G. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem. Toxico. 2006, 44, 1890–1896. [Google Scholar] [CrossRef]
- Shin, C.-Y.; Lee, W.-J.; Lee, E.-B.; Choi, E.-Y.; Ko, K.-H. Platycodin D and D3 Increase Airway Mucin Release in vivo and in vitro in Rats and Hamsters. Planta Med. 2002, 68, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Lee, E.-B. Pharmacological Studies on Platycodon grandiflorum A.DC. I. Acute Toxicity and Central Depressant Activity of Crude Platycodin. Yakugaku Zasshi 1972, 92, 951–960. [Google Scholar]
- Yokoyama, H.; Hiai, S.; Oura, H. Rat Plasma Corticosterone Secretion- inducing Activities of Total Saponin and Prosapogenin Methyl Esters from the Roots of Platycodon grandiflorum A.DC. Yakugaku Zasshi 1982, 102, 1191–1194. [Google Scholar]
- Xu, B.J.; Han, L.K.; Zheng, Y.N.; Lee, J.-H.; Sung, C.-K. In vitro inhibitory of triterpenoidal saponins from platycodi radix on pancreatic lipase. Arch. Pharm. Res. 2005, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Kim, Y.-S. Determination of kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch. Pharm. Res. 2004, 27, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Sim, J.-S.; Shim, S.H.; Ha, Y.W.; Kang, S.S.; Kim, Y.S. Antiobese and hypolipidemic effects of platicodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int. J. Obesity 2005, 29, 983–990. [Google Scholar] [CrossRef]
- Zhao, H.L.; Cho, K.-H.; Ha, Y.W.; Jeong, T.-S.; Lee, W.S.; Kim, Y.S. Cholesterol-lowering effect of platycodin D in hypercholesterolemic ICR mice. Eur. J. Pharmacol. 2006, 537, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Hahn, B.-S.; Kwack, K.B.; Lee, E.B.; Kim, Y.S. Platycodin D-induced apoptosis through nuclear factor-κB activation in immortalized keratinocytes. Eur. J. Pharmacol. 2006, 537, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Noh, E. J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7. Life Sci. 2005, 76, 2315–2328. [Google Scholar] [CrossRef]
- Hara, S.; Okabe, H.; Mihashi, K. Separation of aldose enantiomers by gas-liquid chromatography. Chem. Pharm. Bull. 1986, 34, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-F.; Zhang, S.-J.; Li, N.; Wang, M.-Z.; Sakai, J.; Hasegawa, T.; Mitsui, T.; Kataoka, T.; Oka, S.; Kiuchi, M.; Hirose, K.; Ando, M. Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J. Nat. Prod. 2005, 68, 198–206. [Google Scholar]
- Guido, F.; Pauli. The cardenolide of Speirantha convallarioides. Planta Med. 1995, 61, 162–166. [Google Scholar]
- Sample availability: Available from the author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhang, L.; Liu, Z.-H.; Tian, J.-K. Cytotoxic Triterpenoid Saponins from the Roots of Platycodon grandiflorum. Molecules 2007, 12, 832-841. https://doi.org/10.3390/12040832
Zhang L, Liu Z-H, Tian J-K. Cytotoxic Triterpenoid Saponins from the Roots of Platycodon grandiflorum. Molecules. 2007; 12(4):832-841. https://doi.org/10.3390/12040832
Chicago/Turabian StyleZhang, Lin, Zhen-Huan Liu, and Jing-Kui Tian. 2007. "Cytotoxic Triterpenoid Saponins from the Roots of Platycodon grandiflorum" Molecules 12, no. 4: 832-841. https://doi.org/10.3390/12040832




