Syntheses and Biological Activities of 6-Aryl-3-(3-hydroxy- propyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines
Abstract
:Introduction
Results and Discussion
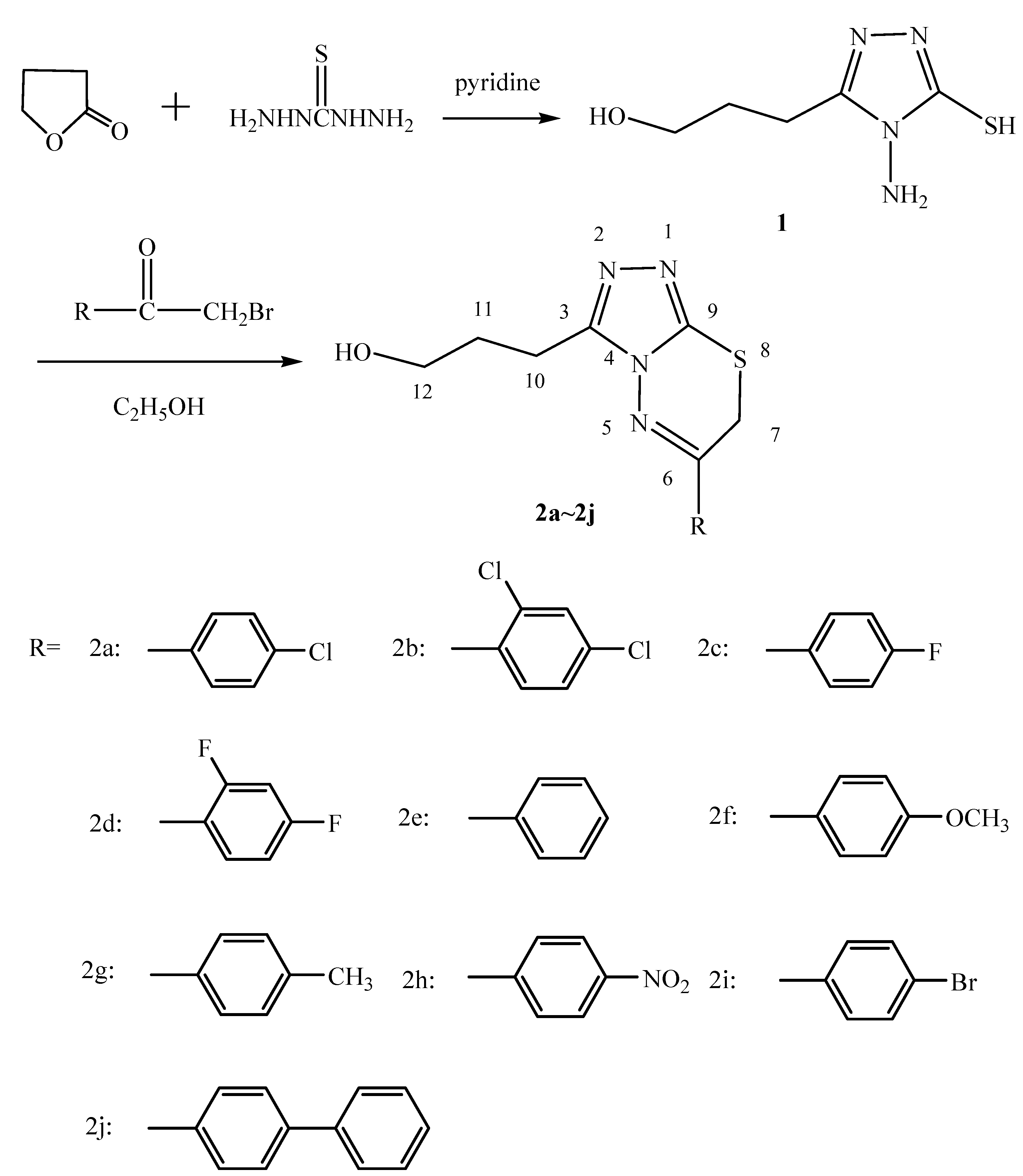
Synthetic route and improvement of synthetic methods
IR spectra of the title compounds
1H-NMR spectra of the title compounds
13C-NMR spectra of the title compounds
Biological Activity

| Compd. | Radish | Wheat | ||
|---|---|---|---|---|
| 10 ppm | 100 ppm | 10 ppm | 100 ppm | |
| 1 | 80/25.6/22.8 | 85/9.7/-12.9 | 70/-20.6 /4.7 | 55/-23.8/-34.1 |
| 2a | 20/-43.2/-50.9 | -100% | 81.3/-68/-68.9 | -100% |
| 2b | -100% | -100% | 85/-94.7/-89.5 | -100% |
| 2c | 20/-42.3/-73.8 | -100% | 94.7/-74.6/-71.9 | -100% |
| 2d | 35/-51.4/-45.6 | -100% | 83.3/-65.9/-64.3 | -100% |
| 2e | 5/-100/-91.3 | -100% | 83.3/-91.3/-84.7 | -100% |
| 2f | 10/-52.7/-84.7 | -100% | 84.2/-87.9/-80.7 | -100% |
| 2g | 10/-38.5/-66.2 | -100% | 100/-92/-87.7 | -100% |
| 2h | 60/-36.3/-42.1 | -100% | 75/-35.4/-44.5 | 95/-96.6/-96.1 |
| 2i | 20/-68.5/-72.7 | -100% | 94.7/-84.3/-77.8 | -100% |
| 2j | 25/-57.7/-74.2 | -100% | 77.8/-87.9/-83.7 | -100% |
| Reference | 85/0/0 | 85/0/0 | 100/0/0 | 100/0/0 |
Experimental
General
Preparation of 4-amino-3-(3-hydroxypropyl)- 3-(3-hydroxypropyl)-5-mercapto-1,2,4-triazole (1)
General method for the preparation of 6-aryl-3-(3-hydroxypropyl)-7H-1,2,4-triazolo[3,4-b][1,3,4] thiadiazines 2a~2j
| Compd. | Molecular formula | Colour | Yield/% | M.p./°C | Anal./% Found (calc.) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 2a | C13H13ClN4OS | white | 76.5 | 134~136 | 50.28(50.57) | 4.09(4.24) | 18.43(18.14) |
| 2b | C13H12Cl2N4OS | yellow | 80.2 | 119~121 | 45.31(45.49) | 3.61(3.52) | 16.05(16.32) |
| 2c | C13H13FN4OS | white | 59.6 | 163~165 | 53.67(53.41) | 4.70(4.48) | 19.44(19.17) |
| 2d | C13H12F2N4OS | yellow | 77.1 | 105~107 | 50.19(50.32) | 3.66(3.90) | 18.37(18.05) |
| 2e | C13H14N4OS | white | 48.5 | 155~157 | 56.80(56.91) | 5.36(5.14) | 20.16(20.42) |
| 2f | C14H16N4O2S | white | 70.3 | 188~190 | 55.37(55.25) | 5.18(5.30) | 18.69(18.41) |
| 2g | C14H16N4OS | white | 55.7 | 144~146 | 58.02(58.31) | 5.72(5.59) | 19.12(19.43) |
| 2h | C13H13N5O3S | brown | 74.1 | 163~165 | 48.64(48.89) | 3.95(4.10) | 22.25(21.93) |
| 2i | C13H13BrN4OS | white | 66.9 | 156~158 | 43.97(44.20) | 3.98(3.71) | 15.68(15.86) |
| 2j | C19H18N4OS | yellow | 72.3 | 175~177 | 65.42(65.12) | 5.33(5.18) | 15.84(15.99) |
| Compd. | IR (ν/cm-1) |
|---|---|
| 2a | 3421 (OH), 2912 (CH2), 1633 (C=N), 1462 (N=C-S), 626 (C-S-C). |
| 2b | 3436 (OH), 2921 (CH2), 1635 (C=N), 1465 (N=C-S), 630 (C-S-C). |
| 2c | 3414 (OH), 2895 (CH2), 1606 (C=N), 1497 (N=C-S), 629 (C-S-C) |
| 2d | 3417 (OH), 2975 (CH2), 1620 (C=N), 1474 (N=C-S), 630 (C-S-C). |
| 2e | 3426 (OH), 2921 (CH2), 1635 (C=N), 1447 (N=C-S), 688 (C-S-C). |
| 2f | 3446 (OH), 2979 (CH2), 1597 (C=N), 1469 (N=C-S), 650 (C-S-C). |
| 2g | 3431 (OH), 2910 (CH2), 1624 (C=N), 1462 (N=C-S), 635 (C-S-C). |
| 2h | 3405 (OH), 2929 (CH2), 1620 (C=N), 1467 (N=C-S), 688 (C-S-C). |
| 2i | 3417 (OH), 2912 (CH2), 1630 (C=N), 1460 (N=C-S), 624 (C-S-C). |
| 2j | 3417 (OH), 2902 (CH2), 1632 (C=N), 1462 (N=C-S), 690 (C-S-C). |
| Compd. | 1H-NMR (δ, ppm) | 13C-NMR (δ, ppm) |
|---|---|---|
| 2a | 1.86~3.53 (m, 6H, C-H), 4.48 (s, 2H, S-CH2), 7.66~8.14 (m, 4H, Ar-H) | 20.77, 22.93, 29.17, 59.71, 129.19, 129.40, 132.00, 137.10, 141.28, 153.78, 155.26 |
| 2b | 1.80~3.51 (m, 6H, C-H), 4.26 (s, 2H, S-CH2), 4.53~4.56 (m, 1H, O-H), 7.61~7.86 (m, 3H, Ar-H) | 20.82, 25.83, 29.52, 59.82, 127.99, 129.76, 132.25, 132.51, 133.45, 136.14, 140.00, 153.66, 154.56 |
| 2c | 1.87~3.53 (m, 6H, C-H), 4.47 (s, 2H, S-CH2), 7.41~8.14 (m, 4H, Ar-H) | 20.75, 22.97, 29.27, 59.73, 116.38, 130.26, 141.20, 153.69, 155.28, 162.76, 166.09 |
| 2d | 1.81~3.50 (m, 6H, C-H), 4.30 s, 2H, S-CH2), 7.28~7.93 (m, 3H, Ar-H) | 21.02, 25.25, 29.67, 60.03, 105.42, 112.63, 119.83, 132.15, 140.09, 151.84, 153.82, 162.70, 165.87 |
| 2e | 1.86~3.53 (m, 6H, C-H), 4.49 (s, 2HS-CH2), 7.56~8.06 (m, 5H, Ar-H) | 20.98, 23.21, 29.35, 59.92, 127.79, 129.29, 132.46, 133.33, 141.68, 153.98, 156.61 |
| 2f | 1.88~3.54 (m, 6H, C-H), 3.86 (s, 3H, CH3), 4.49 (s, 2H, S-CH2), 7.13~8.06 (m, 4H, Ar-H) | 20.78, 22.69, 29.23, 55.57, 59.77, 114.52, 125.23, 129.48, 141.16, 153.50, 155.54,162.49 |
| 2g | 1.84~3.53 (m, 6H, C-H), 2.39 (s, 3H, CH3), 4.36 (s, 2H, S-CH2), 7.36~7.93 (m, 4H, Ar-H) | 20.86, 21.00, 22.79, 29.58, 59.91, 127.32, 129.56, 130.76, 140.01, 142.00, 153.51, 154.45 |
| 2h | 1.85~3.54 (m, 6H, C-H), 4.46 (s, 2H, S-CH2), 8.24~8.42 (m, 4H, Ar-H) | 20.73, 22.92, 29.46, 59.76, 123.95, 128.69, 139.47, 139.79, 148.99, 152.81, 153.73 |
| 2i | 1.84~3.54 (m, 6H, C-H), 4.38 (s, 2H, S-CH2), 4.54~4.57 (m, 1H, O-H), 7.77~7.96 (m, 4H, Ar-H) | 20.75, 22.67, 29.48, 59.79, 125.51, 129.24, 131.95, 132.73, 139.83, 153.53, 153.58 |
| 2j | 1.88~3.53 (m, 6H, C-H), 4.43 (s, 2H, S-CH2), 4.59 (s, 1H, O-H), 7.43~8.12 (m, 9H, Ar-H) | 20.89, 22.83, 29.62, 59.92, 126.84, 127.12, 128.01, 128.28, 129.06, 132.46, 138.80, 139.97, 143.21, 153.59, 154.14 |
Acknowledgments
References
- Chadha, V. K.; Ranwa, N. S.; Dadheech, P. K. Synthesis and screening of biological activity of triazolothiadiazines and triazolothiadiazoles. J. Phytol. Res. 1998, 11, 201–202. [Google Scholar]
- Sakata, M.; Shirakawa, Y.; Kamata, N.; Hiroshino, Y.S.; Jie, O. Y. Synthesis and antibacterial activity of some new s-triazolo[3,4-b]-1,3,4- thiadiazine derivatives. J. Heterocycl. Chem. 2000, 37, 269–271. [Google Scholar]
- Nadkarni, B. A.; Kamat, V. R.; Khadse, B. G. Synthesis and anthelmintic activity of 3,6-disubstituted -7H-s-triazolo[3,4-b][1,3,4] thiadiazines. Arzneim. Forsch. 2001, 51, 569–573. [Google Scholar]
- Holla, B. S.; Akberali, P. M.; Shivananda, M. K. Studies on nitrophenylfuran derivatives: part XII. synthesis, characterization, antibacterial and antiviral activities of some nitrophenyl-furfurylidene- 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Farmaco 2001, 56, 919–927. [Google Scholar] [CrossRef]
- Hui, X. P.; Zhang, Y.; Xu, P. F.; Wang, Q.; Zhang, Q.; Zhang, Z. Y. Syntheses and antibacterial activities of novel 3-substituted-6-(4-chlorophenyl)-7-(1H-1,2,4-triazole-1-yl)-1',2',4'-triazolo [3,4-b]-1",3",4"-thiadiazines. Chin. J. Org. Chem. 2005, 25, 700–704. [Google Scholar]
- Holla, B. S.; Rao, B. S.; Sarojini, B. K.; Akberali, P. M.; Kumari, N. S. Synthesis and studies on some new fluorine containing triazolothiadiazines as possible antibacterial, antifungal and anticancer agents. Eur. J. Med. Chem. 2006, 41, 657–663. [Google Scholar] [CrossRef]
- Sun, X. H.; Liu, Y. F. Studies on synthesis of 1,3-diaminothiourea. Huaxue Tongbao 1999, 62, 43–48. [Google Scholar]
- Awad, L. F.; El Asir, E. S. Synthesis and conformational analysis of seco C- nucleosides and their diseco double-headed analogues of the 1,2,4-triazole, 1,2,4-triazolo[3,4-b]1,3,4-thiadiazole. Carbohyd. Res. 1998, 312, 9–22. [Google Scholar] [CrossRef]
- Zhang, A. J.; Zhang L. X.; Xiong, Y.; Xu, D. J.; Li, X. J. Studies on synthesis and chromatography of 6-Aryl-3-(D-glucopentitol-1-yl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Chin. J. Org. Chem. 2003, 23, 456–460. [Google Scholar]
- Sample Availability: Not available.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jin, J.-y.; Zhang, L.-x.; Chen, X.-x.; Zhang, A.-j.; Zhang, H.-l. Syntheses and Biological Activities of 6-Aryl-3-(3-hydroxy- propyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Molecules 2007, 12, 297-303. https://doi.org/10.3390/12030297
Jin J-y, Zhang L-x, Chen X-x, Zhang A-j, Zhang H-l. Syntheses and Biological Activities of 6-Aryl-3-(3-hydroxy- propyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Molecules. 2007; 12(3):297-303. https://doi.org/10.3390/12030297
Chicago/Turabian StyleJin, Jian-yu, Li-xue Zhang, Xian-xing Chen, An-jiang Zhang, and Hai-le Zhang. 2007. "Syntheses and Biological Activities of 6-Aryl-3-(3-hydroxy- propyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines" Molecules 12, no. 3: 297-303. https://doi.org/10.3390/12030297




