Pongaflavanol: a Prenylated Flavonoid from Pongamia pinnata with a Modified Ring A
Abstract
:Introduction
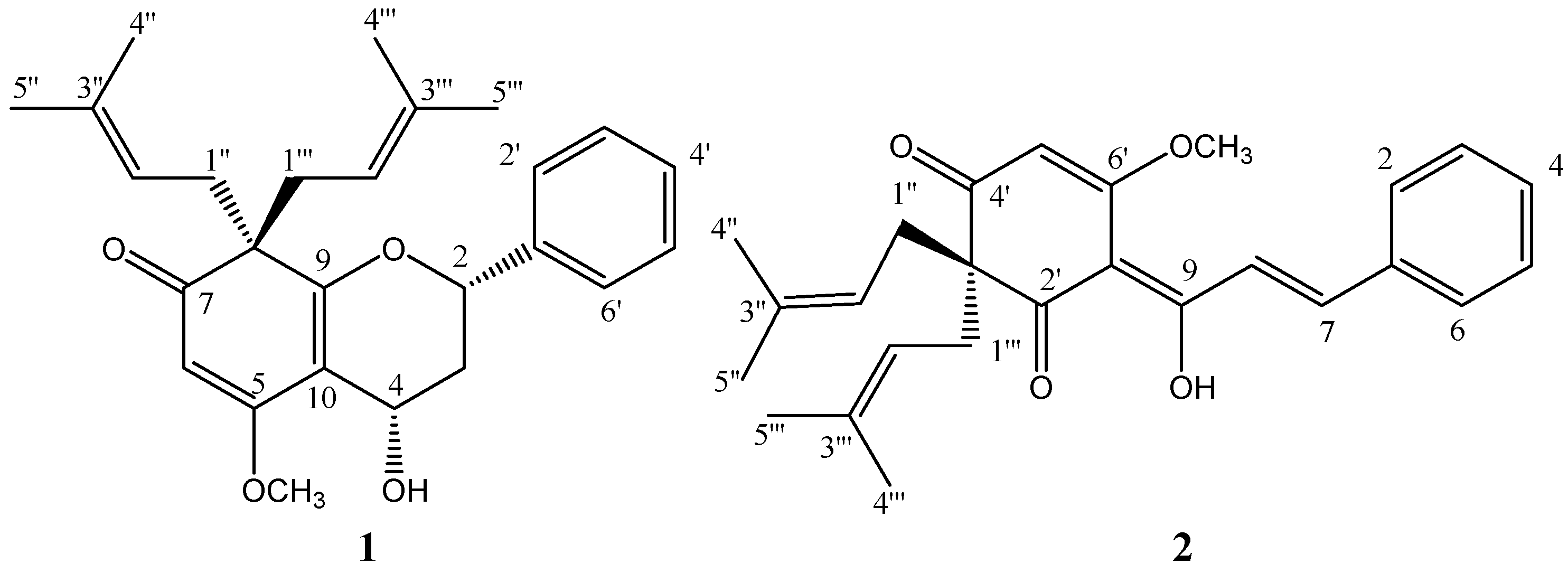
Results and Discussion
| position | δH (J=Hz) | δc | HMBC | H1-H1 COSY | NOESY |
|---|---|---|---|---|---|
| 2 | 4.88 dd (12.2, 1.8) | 77.9 | 3,4,9,1′,2′,6′ | 3 | 3a, 4 |
| 3a | 2.45-2.48 ddd (13.7, 7.2, 1.8) | 37.9 | 4, 10, | 2, 4 | 2, 4 |
| 3b | 2.04-2.12 ddd (13.6, 12.2, 9.4) | 37.9 | 1′, 2, 4 | 2, 4 | 4 |
| 4 | 4.96 dd (9.4, 7.3) | 62.2 | 3, 9, 10 | 3 | 3a, 3b, 2 |
| 5 | 173.5 | ||||
| 6 | 5.41 s | 97.1 | 5, 7, 8, 10 | OCH3 | |
| 7 | 200.1 | ||||
| 8 | 57.2 | ||||
| 9 | 166.7 | ||||
| 10 | 108.4 | ||||
| 1′ | 139.5 | ||||
| 2′, 6′ | 7.33-7.42 m | 125.9 | 2 | ||
| 3′, 5′ | 7.33-7.42 m | 128.6 | |||
| 4′ | 7.33-7.42 m | 128.3 | |||
| 1′′ | 2.55-2.67 m | 38.3 | 7, 8, 9, 1′′′ | 1′′′ | |
| 2′′ | 5.00 t (6.9) | 118.5 | |||
| 3′′ | 134.3 | ||||
| 4′′ | 1.63 s | 18.2 | |||
| 5′′ | 1.64 s | 25.8 | |||
| 1′′′ | 2.54 m | 38.1 | 7, 8, 9, 1′′ | 1′′ | |
| 2′′′ | 4.80 t (6.8) | 118.9 | |||
| 3′′′ | 133.7 | ||||
| 4′′′ | 1.49 s | 18.0 | |||
| 5′′′ | 1.59 s | 25.9 | |||
| OCH3 | 3.85 s | 56.3 | 5 | 6 |

Experimental
General
Plant Material
Extraction and Isolation
Acknowledgements
References and Notes
- Tanaka, T.; Iinuma, M.; Fujii, Y.; Yuki, K.; Mizuno, M. Flavonoids in root bark of Pongamia pinnata. Phytochemistry 1992, 31, 993–998. [Google Scholar] [CrossRef]
- Dahanukar, S. A.; Kulkarni, R. A.; Rege, N. N. Pharmacology of medicinal plants and natural products. Ind. J. Pharmac. 2000, 32, 81–118. [Google Scholar]
- Shoba, F. G.; Thomas, M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhea. J. Ethnopharmacol. 2001, 76, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H. T.; Nordskjold, J. B.; Smitt, U. W.; Nyman, U.; Palpu, P.; Joshi, P.; Varughese, G. In vitro screening of Indian medicinal plants for antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Muruganandan, S.; Chandra, J.; Lal, S.; Tandan, S. K.; Prakash, V. R. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J. Ethnopharmacol. 2001, 78, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Misra, S. K.; Sahu, K. C. Screening of some indigenous plants for antifungal activity against dermatophytes. Ind. J. Pharmac. 1977, 9, 269–272. [Google Scholar]
- Carcache-Blanco, E. J.; Kang, Y. H.; Park, E. J.; Kardono, B.-N.; Su, L. B. S.; Riswan, S.; Fong, H. H. S.; Pezzuto, J. M.; Kinghorn, A. D. Constituents of the Stem Bark of Pongamia pinnata with the potential to induce quinone reductase. J. Nat. Prod. 2003, 66, 1197–1202. [Google Scholar]
- Yadav, P. P.; Ahmad, G. A.; Maurya, R. Furanoflavonoids from Pongamia pinnata fruits. Phytochemistry 2004, 65, 439–443. [Google Scholar]
- Yin, H.; Zhang, S.; Wu, J. Prenylated Flavonoids from Pongamia pinnata. Z. Naturforsch 2005, 60b, 356–358. [Google Scholar]
- Andrei, C.; Ferreira, D. T.; Faccione, M.; de Moraes, L. A.; de Carvalho, M.; Braz-Filho, R. C-prenylflavonoids from Roots of Tephrosia tunicate. Phytochemistry 2000, 55, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Yankep, E.; Mbafor, J. T.; Fomuma, Z. T.; Steinbeck, C.; Messanga, B. B.; Nyasse, B.; Budzikiewicz, H.; Lenz, C.; Schmickler, H. Further Isoflavonoid Metabolites from Millettia griffoniana (Bail). Phytochemistry 2001, 56, 363–368. [Google Scholar]
- Pouget, C.; Fagnere, C.; Basly, J.; Leveque, H.; Chulia, A. Synthesis and Structure of Flavan-4-ols and 4-Methoxyflavans as New Potential Anticancer Drugs. Tetrahedron 2000, 56, 6047–6052. [Google Scholar] [CrossRef]
- Wesley, B.; Otto, F.; Carl, G.; Oswald, C.; Brad, R.; Ben, B. Expression Profiling of the Maize Flavonoid Pathway Genes Controlled by Estradiol-Inducible Transcription Factors CRC. Plant Cell 2000, 12, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of pongaflavanol (1) and tunicatachalcone (2) are available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yin, H.; Zhang, S.; Wu, J.; Nan, H.; Long, L.; Yang, J.; Li, Q. Pongaflavanol: a Prenylated Flavonoid from Pongamia pinnata with a Modified Ring A. Molecules 2006, 11, 786-791. https://doi.org/10.3390/11100786
Yin H, Zhang S, Wu J, Nan H, Long L, Yang J, Li Q. Pongaflavanol: a Prenylated Flavonoid from Pongamia pinnata with a Modified Ring A. Molecules. 2006; 11(10):786-791. https://doi.org/10.3390/11100786
Chicago/Turabian StyleYin, Hao, Si Zhang, Jun Wu, Haihan Nan, Lijuan Long, Jin Yang, and Qingxin Li. 2006. "Pongaflavanol: a Prenylated Flavonoid from Pongamia pinnata with a Modified Ring A" Molecules 11, no. 10: 786-791. https://doi.org/10.3390/11100786
APA StyleYin, H., Zhang, S., Wu, J., Nan, H., Long, L., Yang, J., & Li, Q. (2006). Pongaflavanol: a Prenylated Flavonoid from Pongamia pinnata with a Modified Ring A. Molecules, 11(10), 786-791. https://doi.org/10.3390/11100786




