Spectral Data
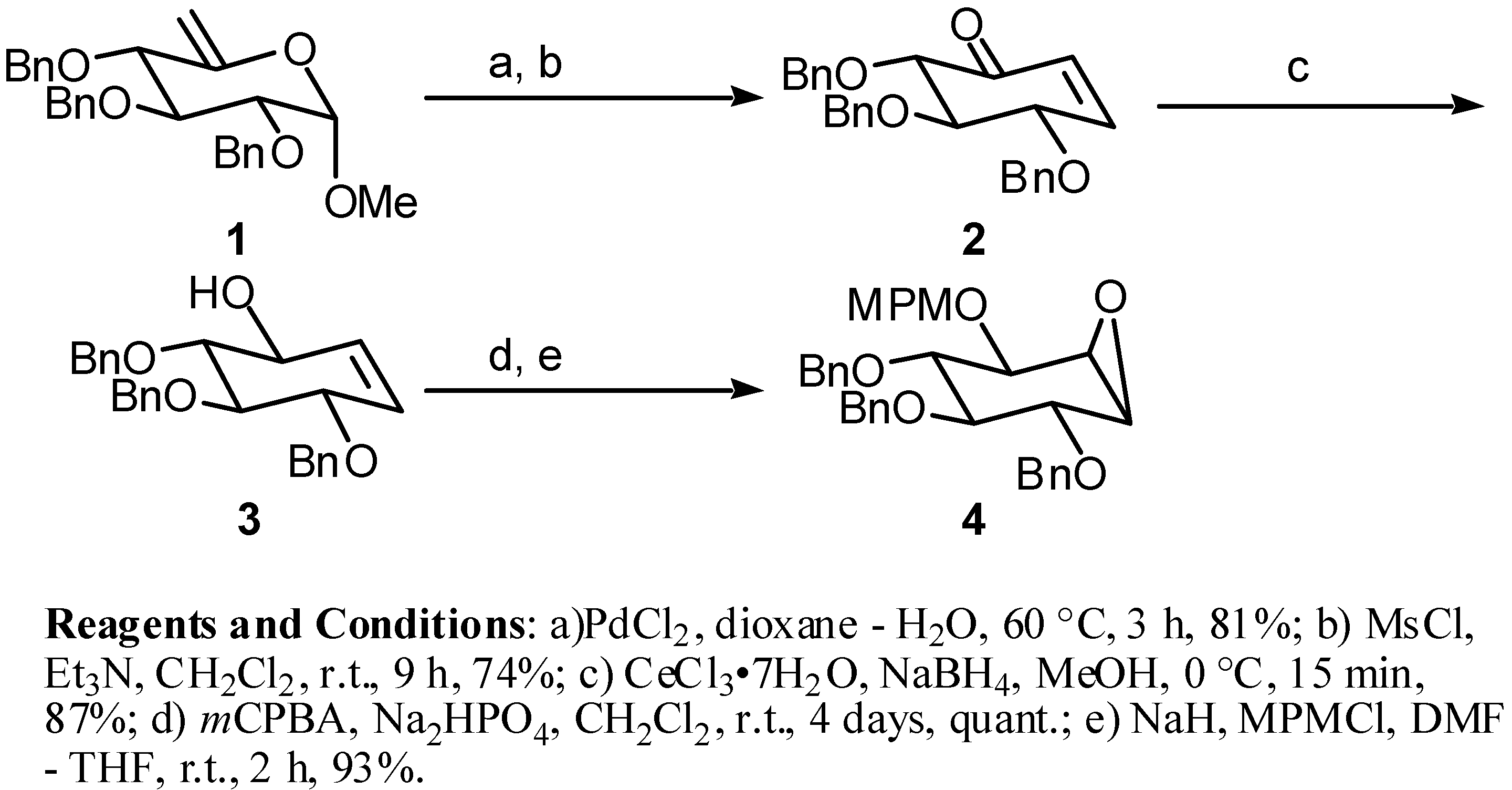
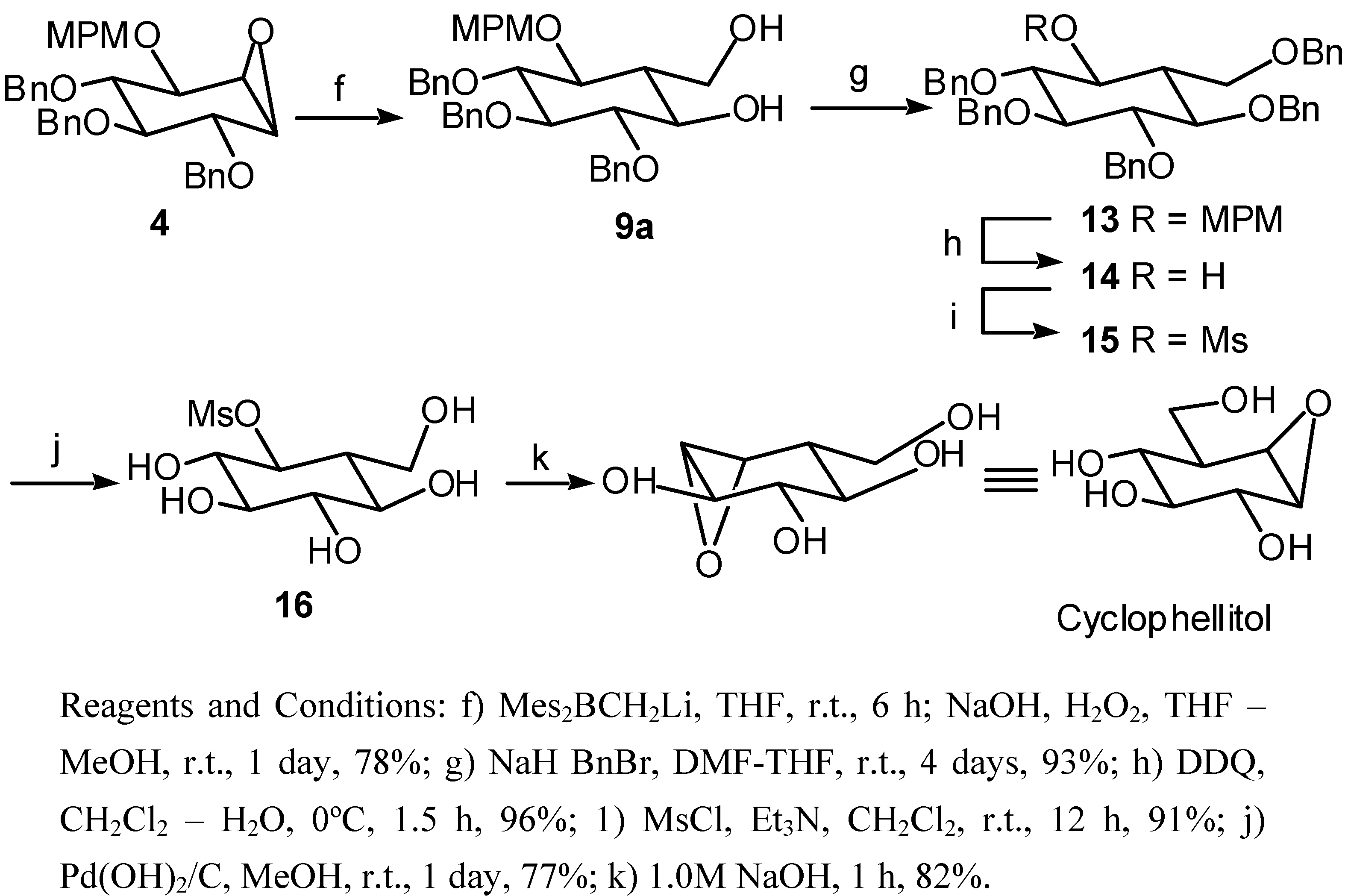
(1S,2R,3S,4S,5R,6R)-2,3,4-Tris(benzyloxy)-5-[(4-methoxybenzyl)oxy]-7-oxabicyclo[4.1.0]heptane (4): 1H-NMR (CDCl3) δ: 7.50-7.24 (m, 17H), 6.90-6.85 (m, 2H), 4.90-4.60 (m, 8H), 3.88 (m, 2H), 3.79 (s, 3H), 3.60 (dd, 1H, J=10.4, 8.5), 3.46 (dd, 1H, J=10.4, 7.9), 3.27 (m, 1H), 3.18 (d, 1H, J=3.7); 13C-NMR (CDCl3) δ: 159.3, 138.6, 137.6, 130.32, 129.5, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 127.6, 127.5, 113.83, 79.0, 76.7, 75.9, 75.5, 73.3, 72.7, 55.2, 53.9; HRMS (EI) for C35H36O6 (M+): Calcd 552.2512; Found 552.2512.; Mp.110°C
(1S,2R,3R,4S,5R,6S)-2,3,4-Tris(benzyloxy)-5-[(benzyloxy)methoxy]-7-oxabicyclo[4.1.0]heptane (6): 1H-NMR (CDCl3) δ: 7.38-7.27 (m, 20H), 5.00 (d, 1H, J=7.0), 4.94 (d, 1H, J=7.0), 4.89-4.59 (m, 6H), 4.07 (1H, dd, J=10.4, 1.8), 3.89 (d, 1H, J=7.9), 3.56 (dd, 1H, J=10.4, 8.5), 3.49 (dd, 1H, J=10.4, 7.9), 3.43 (m, 1H), 3.22 (d, 1H, J=3.7); HRMS (CI) for C35H36O2 (M+): Calcd 552.2512; Found 552.2514.
tert-Butyl(dimethyl)[[(1S,2R,3S,4R,5R,6S)-3,4,5-tris(benzyloxy)-7-oxabicyclo[4.1.0]hept-2-yl]oxy]- silane (7): 1H-NMR (CDCl3) δ: 7.38-7.19 (m, 15H), 4.85-4.64 (m, 6H), 4.09 (m, 1H), 3.90 (m, 1H), 3.46 (m, 2H), 3.25 (m, 1H), 3.18 (d, 1H, J = 4.0), 0.93 (s, 9H), 0.14 (s, 3H), 0.09 (s, 3H); 13C-NMR (CDCl3) δ: 138.8, 138.6, 137.7, 128.5, 128.2, 128.1, 127.9, 127.8, 127.6, 127.5, 127.3, 83.4, 79.7, 79.4, 75.7, 75.5, 73.3, 57.6, 54.0, 25.8, 18.1, -4.5, -4.6; HRMS (EI) for C33H42O5Si (MH+): Calcd 546.2802; Found 546.2802.
(1S,2R,3S,4R,5R,6S)-3,4,5-Tris(benzyloxy)-7-oxabicyclo[4.1.0]hept-2-yl acetate (8): 1H-NMR (CDCl3) δ: 7.40-7.21 (m, 15H), 5.28 (d, 1H, J=8.2), 4.78-4.70 (m, 5H), 4.60 (d, 1H, J=11.6), 3.90 (d, 1H, J=7.3), 3.66-3.56 (m, 2H), 3.40 (m, 1H), 3.23 (d, 1H, J=3.7), 2.02 (s, 3H); 13C-NMR (CDCl3) δ: 170.5, 138.3, 137.4, 128.5, 128.3, 128.0, 127.8, 127.6, 83.2, 78.9, 75.6, 75.5, 73.6, 73.2, 54.6, 54.0, 20.9l; HRMS (EI) for C29H30O6 (M+): Calcd 474.2043; Found 474.2044.
(1R,2S,3R,4R,5S,6R)-2,3,4-Tris(benzyloxy)-6-(hydroxymethyl)-5-[(4-methoxybenzyl)oxy]cyclohexanol (9a): 1H-NMR (CDCl3) δ: 7.38-7.26 (m, 15H), 7.21 (m, 2H), 6.85 (m, 2H), 4.98 (d, 1H, J=11.6), 4.93-4.81 (m, 5H), 4.68 (d, 1H, J=11.3), 4.56 (d, 1H, J=10.7), 3.89 (dd, 1H, J=10.7, 3.1), 3.77 (s, 3H), 3.74 (dd, 1H, J=10.7, 4.7), 3.63 (dd, 1H, J=9.2, 9.2), 3.49 (m, 2H), 3.39 (m, 2H), 1.65 (m, 1H); 13C-NMR (CDCl3) δ:152.6, 138.7, 138.6, 138.5, 130.4, 130.1, 129.9, 128.9, 128.7, 128.2, 128.1, 128.0, 127.9, 127.8, 114.2, 80.9, 79.3, 77.6, 77.5, 76.0, 75.9, 75.7, 75.2, 61.0, 47.5, 40.9; HRMS (EI) for C36H40O7 (M+): Calcd 584.2774; Found 584.2771; Mp. 131°C
(1R,2S,3R,4R,5S,6R)-2,3,4,5-Tetrakis(benzyloxy)-6-(hydroxymethyl)cyclohexanol (10a): 1H-NMR (CDCl3) δ: 7.36-7.26 (m, 20H), 5.01-4.84 (m, 6H), 4.70-4.62 (m, 2H), 3.91 (m, 1H), 3.75 (m, 1H), 3.66 (dd, 1H, J=9.5, 9.5), 3.51 (m, 2H), 3.40 (m, 2H), 1.68 (m, 1H); 13C-NMR (CDCl3) δ: 138.4, 138.3, 138.1, 128.7, 128.5, 128.4, 128.1, 128.0, 127.9, 127.8, 127.7, 127.6, 86.3, 85.1, 83.2, 77.5, 77.2, 75.7, 75.5, 75.3, 70.6, 60.5, 46.1; MS (EI) for C35H38O6 (M+) : Calcd 554.2668; Found 554.2654
(1R,2R,3R,4R,5S,6R)-2,3,4-Tris(benzyloxy)-5-[(benzyloxy)methoxy]-6-(hydroxymethyl)cyclohexanol (11a): 1H-NMR (CDCl3) δ: 7.40-7.22 (m, 20H), 5.20-4.70 (m, 9H), 4.53 (d, 1H, J=11.9), 3.94 (m, 1H), 3.85 (m, 1H), 3.60-3.36 (m, 5H), 1.57 (m, 1H); 13C-NMR (CDCl3) δ: 138.5, 138.3, 137.0, 128.6, 128.4, 128.0, 127.9, 127.8, 127.7, 127.6, 97.1, 85.8, 85.5, 83.1, 76.5, 75.7, 75.6, 75.4, 70.7, 69.1, 58.0, 46.0; HRMS (EI) for C29H33O7: Calcd 493.2226; Found 493.2226.
(1S,2S,3S,4R,5R,6S)-3,4,5-Tris(benzyloxy)-2-[(benzyloxy)methoxy]-6-(hydroxymethyl)cyclohexanol (11b): 1H-NMR (CDCl3) δ: 7.40-7.22 (m, 20H), 5.05-4.55 (m, 10H), 4.18 (m, 1H), 4.00-3.50 (m, 6H), 2.58 (m, 1H); HRMS (EI) for C29H33O7: Calcd 493.2226; Found 493.2226.
(1S,2S,3S,4R,5R,6S)-3,4,5-Tris(benzyloxy)-2-{[tert-butyl(dimethyl)silyl]oxy}-6-(hydroxymethyl)cyclo-hexanol (12b): 1H-NMR (CDCl3) δ: 7.40-7.21 (m, 15H), 4.86-4.64 (m, 6H), 4.11 (dd, 1H, J=8.8, 5.9), 3.87 (m, 2H), 3.80 (dd, 1H, J=8.8, 8.8), 3.75 (dd, 1H, J=8.8, 2.9), 3.69 (dd, 1H, J=8.8, 8.8), 3.60 (dd, 1H, J=11.0, 4.8), 2.59 (dddd, 1H, J=8.1, 4.8, 5.9, 2.9), 0.90 (s, 9H), 0.08 (s, 3H), 0.06 (s, 3H); 13C-NMR (CDCl3) δ: 138.8, 138.7, 138.0, 131.4, 128.5, 128.3, 128.2, 127.9, 127.8, 127.5, 127.4, 125.8, 82.4, 82.3, 80.1, 76.7, 75.6, 73.9, 73.8, 72.9, 61.4, 43.0, 25.8, 15.6, -4.5, -4.8; HRMS (EI) for C34H46O6Si: Calcd 578.3064; Found 578.3063.
(1S,2R,3S,4S,5R,6S)-2,3,4,5-Tetrakis(benzyloxy)-6-[(benzyloxy)methyl]cyclohexanol (14): 1H-NMR (CDCl3+D2O) δ: 7.33-7.26 (m, 25H), 5.00-4.44 (m, 10H), 3.82 (dd, 1H, J=8.8, 2.2), 3.70 (m, 2H), 3.62 (m, 2H), 3.53 (m, 1H), 3.40 (dd, 1H, J=9.2, 9.2), 1.69 (m, 1H); HRMS (EI) for C42H44O6 (M+): Calcd 644.3138; Found 644.3130.
(1S,2S,3R,4S,5R,6R)-2,3,4,5-Tetrakis(benzyloxy)-6-[(benzyloxy)methyl]cyclohexylmethanesulfonate (15): 1H-NMR (CDCl3) δ: 7.40-7.10 (m, 25H), 5.10-4.35 (m, 11H), 3.87 (m, 1H), 3.75 (m, 1H), 3.67 (m, 1H), 3.65-3.56 (m, 3H), 2.80 (s, 3H), 1.85 (m, 1H); HRMS (EI) for C36H39O8S (M+): Calcd 631.2366; Found 631.2365.
(1S,2S,3R,4S,5R,6R)-2,3,4,5-Tetrahydroxy-6-(hydroxymethyl)cyclohexylmethanesulfonate (16): 1H-NMR (D2O) δ: 4.70 (dd, 1H, J=9.9, 9.9), 3.98 (d, 1H, J=12.0), 3.79 (d, 1H, J=12.0), 3.67 (dd, 1H, J=9.2, 9.2), 1H), 3.34 (m, 4H), 1.82 (dd, 1H, J=11.0, 11.0); HRMS (CI) for C13H24NO6 (MH+): Calcd 290.160363; Found 290.160300.
Cyclophellitol: 1H-NMR (D2O) δ: 4.01 (dd, 1H, J=11.0, 4.0), .83 (dd, 1H, J=11.0, 7.3), 3.79 (d, 1H, J=8.4), 3.56 (dd, 1H, J=4.0, 1.8), 1H), 3.38 (dd, 1H, J=10.3, 8.4), 3.27 (m, 2H), 2.13 (m, 1H); 13C-NMR (D2O) δ: 77.0, 71.6, 67.5, 61.2, 57.0, 56.7l; MS (EI) for C7H12O5 (M+): 176, (M+-H2O): 158.
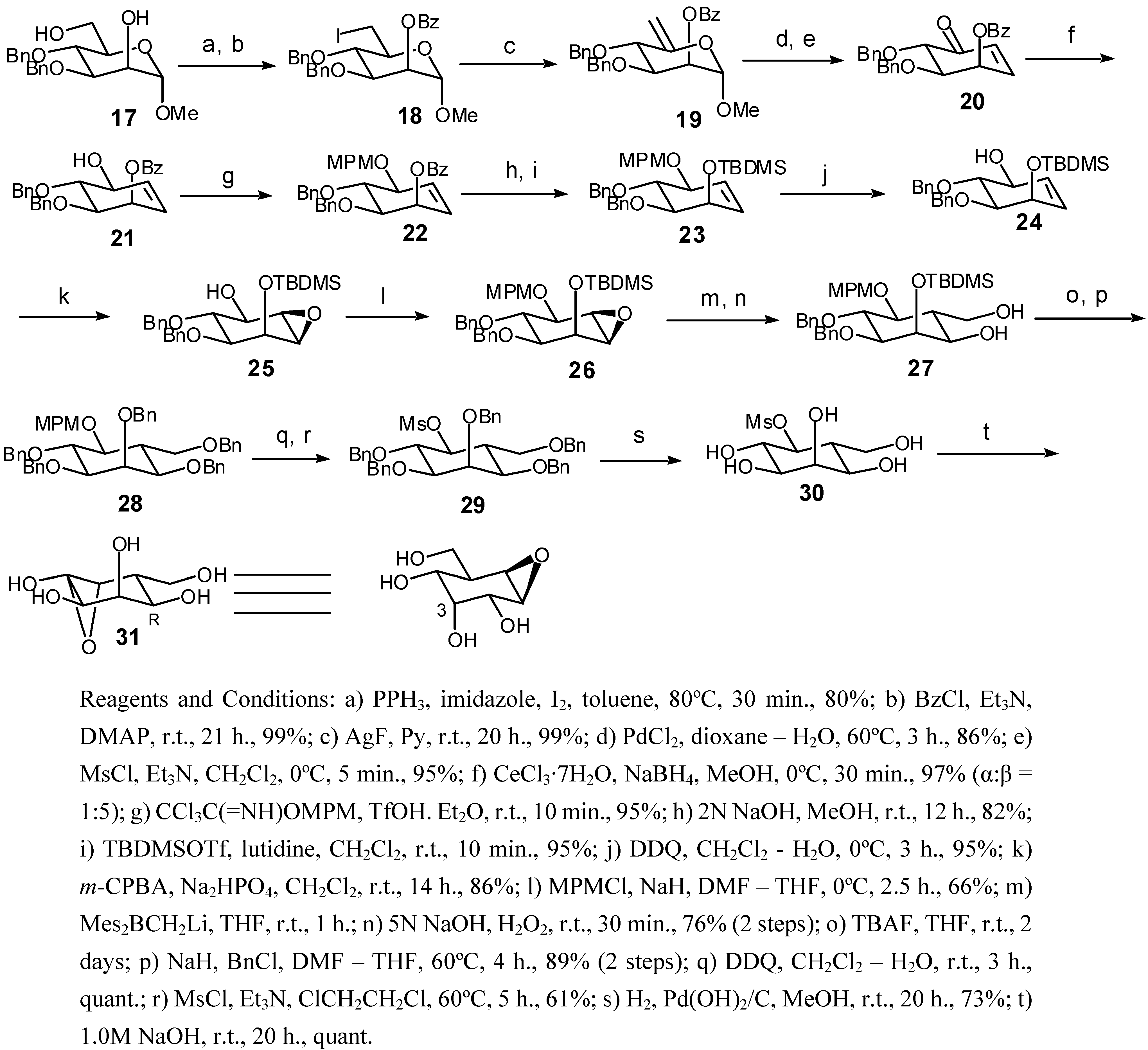
Methyl 2-O-benzoyl-3,4-di-O-benzyl-6-deoxy-6-iodo-α-d-mannopyranoside (18): 1H-NMR (CDCl3) δ: 8.18-8.14 (m, 2H), 7.70-7.23 (m, 13H), 5.62 (m, 1H), 4.96 (d, 1H, J=10.7), 4.83 (d, 1H, J=1.8), 4.77 (d, 1H, J=11.3), 4.69 (d, 1H, J=10.7), 4.55 (d, 1H, J=11.3), 4.11 (dd, 1H, J=9.5, 1.3), 3.82 (dd, 1H, J=9.5, 9.5), 3.59-3.46 (m, 3H), 3.42 (s, 3H); IR (thin film) cm-1: 2954 (broad), 1736, 1611, 1524, 1439, 1323, 1204; HRMS (EI) for C28H29O6I (M+): Calcd 588.1009; Found 588.1022.
Methyl 2-O-benzoyl-3,4-di-O-benzyl-6-deoxy -α-d-lyxo-hex-5-enopyranoside (19): 1H-NMR (CDCl3) δ: 8.08-8.04 (m, 2H), 7.61-7.22 (m, 13H), 5.62 (dd, 1H, J=5.5, 3.0), 4.94 (brs, 1H ), 4.88 (brs, 1H), 4.84-4.61 (m, 5H, J=11.3), 4.36 (d, 1H, J=9.5), 4.05 (dd, 1H, J=9.5, 3.0); 13C-NMR (CDCl3) δ: 170.4, 166.0, 130.2, 130.1, 128.9, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 106.3, 84.8, 81.2, 77.4, 77.2, 72.4, 60.4; HRMS (EI) for C28H28O6 (M+) : Calcd 460.1886; Found 460.1876.
(1R,5S,6R)-5,6-Bis(benzyloxy)-4-oxocyclohex-2-en-yl benzoate (20): 1H-NMR (CDCl3) δ: 8.02-8.00 (m, 2H), 7.65-7.21 (m, 13H), 6.90 (dd, 1H, J=10.4, 4.0), 6.17 (d, 1H, J=10.4 ),6.07 (dd, 1H, J=3.7, 3.7), 4.88 (d, 1H, J=11.9), 4.70 (m, 3H), 4.24 (d, 1H, J=7.6), 4.19 (dd, 1H, J=7.6, 3.4); 13C-NMR (CDCl3) δ: 165.7, 143.6, 138.6, 136.8, 133.2, 128.3, 127.7, 126.5, 125.6, 86.6, 77.0, 76.9, 71.4, 69.5; HRMS (EI) for C27H24O5 (M+): Calcd 428.1624; Found 428.1639.
(1R,4S,5R,6R)-5,6-Bis(benzyloxy)-4-hydroxycyclohex-2-en-1-yl benzoate (21): 1H-NMR (CD3OD) δ: 7.95 (m, 2H), 7.56-7.11 (m, 13H), 5.89-5.80 (m, 3H), 4.78-4.50 (m, 4H), 4.15 (d, 1H, J=7.3), 3.84 (dd, 1H, J=10.3, 7.3), 3.69 (dd, 1H, J=10.3, 3.7); HRMS (CI) for C13H24NO6 (MH+): Calcd 290.160363; Found 290.160300.
(1R,4S,5R,6R)-5,6-Bis(benzyloxy)-4-[(4-methoxybenzyl)oxy]cyclohex-2-en-1-yl benzoate (22): 1H-NMR (CDCl3) δ: 7.95 (m, 2H), 7.40-7.10 (m, 12H), 6.85 (m, 2H), 5.86 (m, 1H), 5.80 (m, 2H), 4.90 (1H, d, J=11.0), 4.76 (1H, d, J=11.0), 4.70 (1H, d, J=11.6), 4.63 (1H, d, J=11.0), 4.56 (2H,m), 4.39 (3H, s), 4.05 (1H,m), 3.70 (2H,m); 13C-NMR (CDCl3) δ: 166.0, 159.2, 138.8, 138.4, 131.4, 130.1, 129.8, 129.7, 129.5, 128.5, 128.3, 127.7, 114.1, 114.0, 79.2, 78.7, 75.4, 68.7, 67.2, 64.6, 62.9, 55.5; HRMS (CI) for C13H24NO6 (MH+): Calcd 290.160363; Found 290.160300.
[[(1R,4S,5R,6S)-5,6-Bis(benzyloxy)-4-[(4-methoxybenzyl)oxy]cyclohex-2-en-1-yl]oxy](tert-butyl)-dimethylsilane (23): 1H-NMR (CDCl3) δ: 7.40-7.20 (m, 12H), 6.85 (m, 2H), 5.77-5.67 (m, 2H), 4.92 (d, 1H, J=11.0 ), 4.79 (d, 1H, J=11.0), 4.73 (s, 2H), 4.59 (d, 1H, J=11.3), 4.54 (d, 1H, J=11.3), 4.32 (dd, 1H, J=8.2, 4.9), 4.22-4.02 (m, 2H), 3.80 (s, 3H), 3.40 (dd, 1H, J=9.8, 4.4), 0.88 (s, 9H), 0.08 (3H,s), 0.05 (3H, s); MS (EI) for C34H44O5Si (M+) 560, (M+-tBu) 503.
(1S,4R,5S,6R)-5,6-Bis(benzyloxy)-4-[[tert-butyl(dimethyl)silyl]oxy]cyclohex-2-en-1-ol (24): 1H-NMR (CDCl3) δ: 7.38-7.26 (m, 10H), 5.78 (dd, 1H, J=10.4, 3.4), 5.70 (dd, 1H, J=10.4, 3.1), 4.82 (d, 1H, J=11.9 ), 4.74-4.61 (m, 3H), 4.52 (m, 1H), 4.01 (m, 1H), 3.86 (dd, 1H, J=6.7, 4.3), 3.64 (dd, 1H, J=6.7, 3.5), 0.93 (s, 9H), 0.10 (s, 3H), 0.09 (3H, s); 13C-NMR (CDCl3) δ: 159.3, 143.0, 138.5, 129.9, 128.9, 128.7, 128.6, 128.1, 128.0, 127.9, 123.7, 121.8, 79.2, 79.0, 78.1, 77.4, 73.5, 72.6, 69.4, 54.3, 26.1, 22.3, -4.3; HRMS (EI) for C26H36O4Si (M+): Calcd 440.2383; Found 440.2383.
(1R,2R,3S,4S,5S,6R)-3,4-Bis(benzyloxy)-5-[[tert-butyl(dimethyl)silyl]oxy]-7-oxabicyclo[4.1.0]heptan- 2-ol (25): 1H-NMR (CDCl3) δ: 7.38-7.18 (m, 10H), 4.78 (d, 1H, J=11.9), 4.54 (m, 3H), 4.36 (dd, 1H, J=4.0, 3.1), 3.96 (m, 1H), 3.65 (dd, 1H, J=4.0, 3.1), 3.56 (dd, 1H, J=4.0, 4.0), 3.48 (dd, 1H, J=4.0, 4.0), 2.86 (d, 1H, J=10.1), 0.96 (s, 9H), 0.15 (s, 3H), 0.13 (3H, s); 13C-NMR (CDCl3) δ:137.8, 128.6, 128.3, 128.1, 127.7, 127.5, 80.8, 76.8, 76.6, 73.6, 72.8, 67.8, 55.7, 54.3, 25.7, 19.6, -4.8; HRMS (EI) for C26H36O5Si(M+): Calcd 456.2332; Found 456.2327.
[[(1R,2S,3S,4R,5R,6R)-3,4-Bis(benzyloxy)-5-[(4-methoxybenzyl)oxy]-7-oxabicyclo[4.1.0]hept-2-yl]-oxy](tert-butyl)dimethylsilane (26): 1H-NMR (CDCl3) δ: 7.40-7.20 (m, 12H), 6.92-6.82 (m, 2H), 4.88-4.62 (m, 6H), 4.31 (dd, 1H, J=4.6, 4.6), 3.89 (dd, 1H, J=10.1, 8.2), 3.80 (s, 3H), 3.74 (dd, 1H, J=8.2, 2.1), 3.22 (dd, 1H, J=4.5, 2.1), 3.19 (dd, 1H, J=4.5, 4.5), 3.14 (dd, 1H, , J=10.1, 4.6), 0.94 (s, 9H), 0.13 (3H, s), 0.11 (3H, s); 13C-NMR (CDCl3) δ: 157.7, 137.9, 131.0, 129.5, 1283.2, 127.6, 127.4, 127.3, 125.9, 122.7, 122.3, 76.9, 76.8, 76.7, 76.6, 70.0, 65.9, 58.8, 57.5, 50.4, 25.8, 19.7, -4.5; HRMS (EI) for C34H44O6Si (M+): Calcd 576.2907; Found 576.2907.
(1R,2R,3S,4R,5S,6R)-3,4-Bis(benzyloxy)-2-[[tert-butyl(dimethyl)silyl]oxy]-6-(hydroxymethyl)-5-[(4- methoxybenzyl)oxy]cyclohexanol (27): 1H-NMR (CDCl3) δ: 7.40-7.20 (m, 12H), 6.90-6.83 (m, 2H), 4.98 (d, 1H, J=11.0), 4.84 (m, 2H), 4.70 (s, 2H), 4.55 (d, 1H, J=11.0), 4.15 (dd, 1H, J=2.1, 2.1), 3.94 (dd, 1H, J=9.8, 9.8), 3.87 (dd, 1H, J=10.7, 3.7), 3.81 (m, 4H), 3.43 (m, 1H), 3.31 (dd, 1H, J=11.0, 9.8), 3.25 (dd, 1H, J=9.8, 2.1), 2.05 (2H, brs), 1.96 (1H, m); 13C=NMR (CDCl3) δ: 159.6, 138.6, 133.2, 129.2, 128.6, 128.5, 128.2, 128.1, 128.0, 127.8, 127.7, 114.2, 111.2, 78.6, 78.1, 77.0, 75.6, 75.1, 73.6, 72.1, 69.9, 61.4, 44.7, 26.3, 18.7, -4.8; HRMS (EI) for C35H48O7Si(M+): Calcd 608.3169; Found 608.3169.
1-Methoxy-4-[[(1S,2R,3R,4R,5R,6R)-2,3,4,5-tetrakis(benzyloxy)-6-[(benzyloxy)methyl]-2-cyclohexyl]- oxymethyl]benzene (28): 1H-NMR (CDCl3) δ: 7.40-7.20 (m, 27H), 7.12 (d, 1H, J=8.5), 6.80 (d, 1H, J=8.5), 4.96 (d, 1H, J=10.7), 4.82 (m, 4H), 4.64 (m, 2H), 4.49 (m, 2H), 4.38 (m, 2H), 4.07 (m, 2H), 3.76 (s, 3H), 3.63 (dd, 1H, J=9.2, 9.2), 3.47 (dd, 1H, J=11.6, 1.8), 3.32 (dd, 1H, J=9.8, 1.8), 2.31 (1H, m); HRMS (EI) for C43H45O7 (M+): Calcd 673.3166; Found 673.3165.
(1S,2S,3R,4R,5R,6R)-2,3,4,5-Tetrakis(benzyloxy)-6-[(benzyloxy)methyl]cyclohexylmethanesulfonate (29): 1H-NMR (CDCl3) δ: 7.40-7.16 (m, 25H), 5.09 (dd, 1H, J=11.3), 4.82 (dd, 1H, J=11.0, 11.0), 4.69-4.35 (m, 9H), 4.12 (dd, 1H, J=9.5, 9.5), 4.03 (brs, 1H), 3.88 (dd, 1H, J=9.5, 2.5), 3.65 (dd, 1H, J=9.5, 1.5), 3.50 (dd, 1H, J=11.3, 2.1), 3.36 (dd, 1H, J=11.3, 2.1), 2.81 (s, 3H), 2.45 (m, 1H); HRMS (EI) for C43H46O8S (M+): Calcd 722.2914; Found 722.2916.
(1S,2S,3R,4R,5R,6R)-2,3,4,5-Tetrahydroxy-6-(hydroxymethyl)cyclohexylmethanesulfonate (30): HRMS (CI) for C13H24NO6 (MH+): Calcd 290.160363; Found 290.160300.
(1S,2R,3R,4R,5R,6R)-5-(Hydroxymethyl)-7-oxabicyclo[4.1.0]heptane-2,3,4-triol (31): 1H-NMR (D2O) δ: 3.85 (m, 1H), 3.82 (m, 2H), 3.65 (m, 1H), 3.43 (bs, 1H), 3.28 (d, 1H, J=9.5), 3.07 (m, 1H), 2.14 (m, 1H); 13C-NMR (D2O) δ: 71.0, 67.4, 66.2, 60.5, 56.3, 55.4, 38.5; HRMS (CI) for C13H24NO6 (MH+): Calcd 290.160363; Found 290.160300.