Introduction
The problems of relativistic thermodynamics and statistics attracted significant attention recently [
1,
2,
3,
4]. The interest to the field was boosted by modern astrophysics investigations. One of the most debated problems is the relativistic temperature transformation. The Planck-Einstein (PE) approach to the temperature transformation resulted in the well-known relation [
5]:
where
T and d
Q are the temperature and the heat respectively. Ott in his early work concluded that the relativistic temperature transformation is at least ambiguous, because the energy transfer from one relativistic system could be carried out in two ways: under the constant velocity and under the constant momentum which are not proportional in the relativity theory [
6]. Since that the relativistic temperature transformation was debated extensively [
7,
8,
9,
10,
11,
12,
13]. F. Angulo-Brown
et al in his recent extended study of the problem have summarized the attempts to obtain the relativistic temperature transformation in such a way:
where
a is the Balescu parameter which is not known with certainty and
a = –1, if the PE transformation is used, and
a = 1 under the Ott considerations [
1,
6,
8,
11]. The idea that the temperature might be invariant was also suggested, thus the situation
a = 0, could be considered as well [
12].
On the other hand Landsberg and Matsas in their recent study asserted that a universal continuous Lorentz transformation of temperature cannot exist [
9,
10]. This was concluded on theoretical grounds that an observer moving in a heat reservoir could not detect a blackbody spectrum. In our present letter we supply additional arguments holding the viewpoint that the Lorentz transformation of the temperature does not exist.
Entropy of Ideal Gas and Temperature Relativistic Transformation
One of the basic arguments supporting PE temperature-heat transformations (1)–(2) resided in the fact that they remain the entropy of the thermodynamic system constant:
Indeed, the entropy defined according to
S = ln
W (we use the system of units in which the Boltzmann constant
kB = 1 [
14,
15]), is a dimensionless value and has to be invariant relatively to the transformations of frames of reference [
5]. We will demonstrate that actually situation is more complicated and even the entropy of mono-atomic ideal gas is not constant under PE transformations.
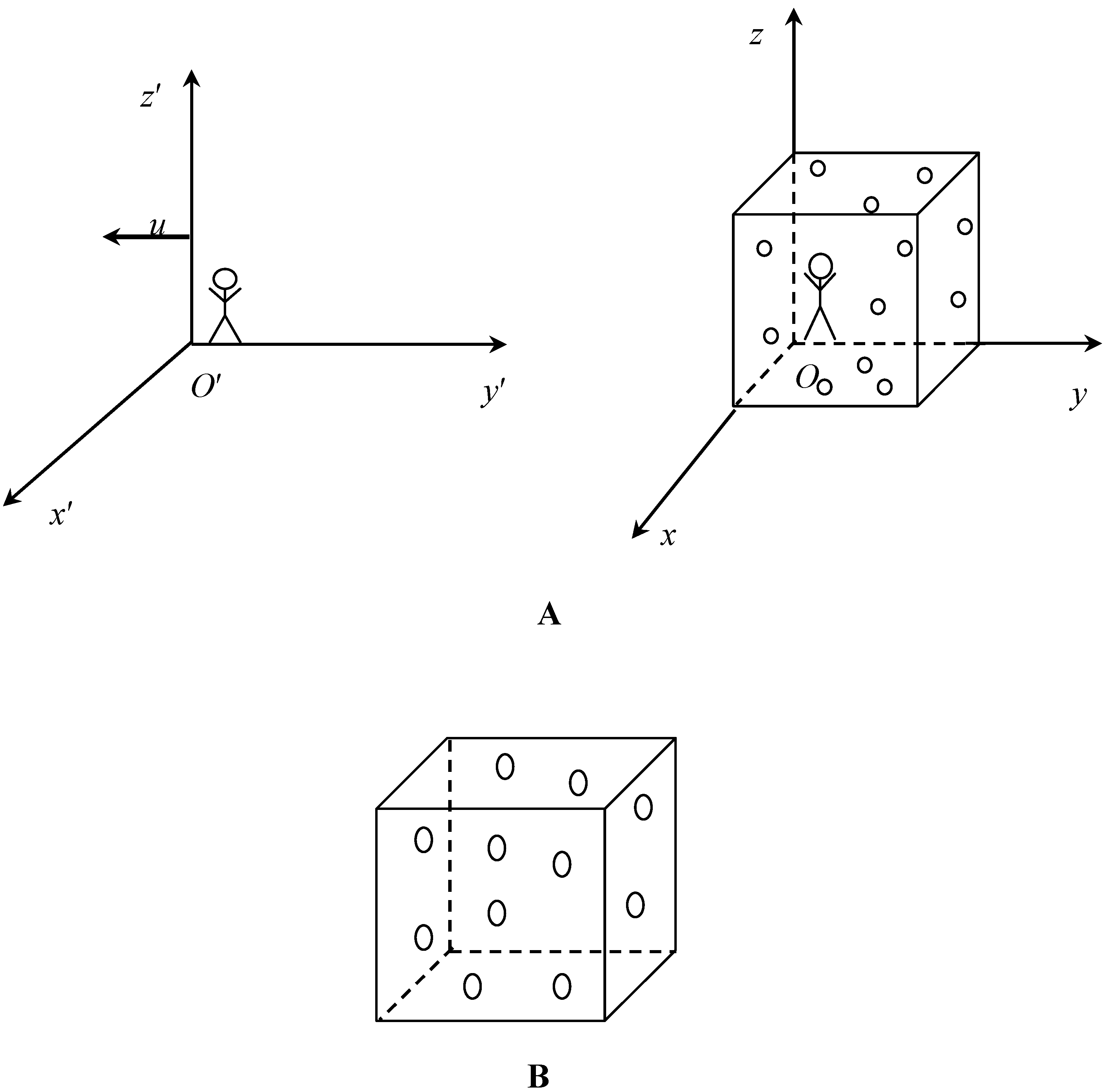
Let us consider the ideal gas, comprising ideal identical spherical atoms (for example Helium or Argon) confined within a vessel from the viewpoint of the observer in frame
XYZ which rests relatively to the vessel (
Figure 1 A). An interaction between atoms is neglected, and in this meaning the gas is ideal. The entropy of the gas for this observer is given by:
where
N and
c are the number and particles concentration respectively,
i –number of degrees of freedom of the particle (
i = 6 for spheres) [
5]. Actually it is established experimentally that the molar heat capacity of Helium, Neon and Argon
Cv equals (3/2)
R in a broad range of at temperatures, with a high accuracy, thus, actually for these gases
i = 3 [
16]. Hence it is clear that rotational degrees of freedom are not excited for monoatomic gases. The reason for this could not be explained in the realm of classical physics, but instead requires quantum mechanics arguments discussed below.
I will restrict the treatment with a classical (non-quantum) monoatomic relativistic gas comprising ideal spherical particles with
i = 6. The entropy of the same gas established by the observer in frame
X'Y'Z' moving with velocity
u, may be changed for several reasons. First of all, the entropy depends on the temperature and concentration of the particles. However, PE transformation provides:
S'(
c',
T') =
S(
c,
T) when
i = const [
5]. For our discussion it is much more important that
S =
S(
i), and the entropy will be changed as a result of the change in the quantity of degrees of freedom of the particle.
The change of
i is stipulated by the fact, that the moving observer will see the gas of ellipsoids (as depicted in
Figure 1B) and not on ideal spheres, (due to the relativistic length contraction), thus for this observer
i = 5 (three translational and two rotational degrees of freedom, because the orientation of ellipsoids central axis is fixed), and
i =
i(
γ(
u)). Hence the entropy turns out to be sensitive to the frames of reference transformations:
S =
S(
γ(
u)).
It has to be emphasized that involving quantum mechanics arguments comes to help and remedies the paradox. Indeed entropy defined according to the well-known Sackur-Tetrode formula still depends on the number of degrees of freedom of the particle:
S =
S(
i) [
14,
15]. However the rotational degrees of freedom will be excited only for temperatures greater than
where
h is the Planck's constant,
I is the moment of inertia of the particle (the Boltzmann constant
kB = 1). These temperature are unrealistically high for real gaseous systems, thus for monoatomic gases
i =3 for both spherical and ellipsoidal particles, hence
S turns out to be insensitive to the frames of reference transformations
There exist several ways to surmount the difficulty under discussion within frameworks of special relativity: 1) to assume that the PE transformation and transformations (3)–(4) are applicable for point particles only, thus no realistic thermodynamic system could be described with them; 2) to suggest that the quantity of degrees of freedom is somewhat like “magic number” and it is insensitive to the transformations of frames of references; 3) to introduce artificial relativistic temperature transformation , taking into account change in the degrees of freedom of the particles; 4) to assume that there is no universal relativistic temperature transformation.
We incline to suppose that the last suggestion coinciding with the approach reported recently by Landsberg [
9,
10] is true. Indeed relativistic temperature transformation is faced with multiple principle difficulties, already described, which seem to be insuperable. On the other hand invoking quantum mechanics arguments brings to the conclusion that number of atomic degrees of freedom is insensitive to the frames of reference transformations for monoatomic systems. Thus it could be concluded that consistent classical relativistic thinking becomes impossible in this case.
Figure 1.
Scheme illustrating entropy jump stipulated bythe change in the number of degrees of freedom.
Figure 1.
Scheme illustrating entropy jump stipulated bythe change in the number of degrees of freedom.