Large Eddy Simulation and Thermodynamic Design of the Organic Rankine Cycle Based on Butane Working Fluid and the High-Boiling-Point Phenyl Naphthalene Liquid Heating System
Abstract
:1. Introduction
1.1. The Organic Rankine Cycle
1.2. Advantages of Applying High-Boiling Liquids
- (1)
- They operate reliably under atmospheric pressure;
- (2)
- The heating temperature can be varied easily;
- (3)
- They are safer to use (steam explosion hazard may be prevented);
- (4)
- Heavy forgings for pressure vessels and piping are eliminated;
- (5)
- They are compatible with low-cost materials (plain carbon steel and aluminum alloys can be applied) and have no corrosion potential.
Boiling Regimes of Water
2. Materials and Methods
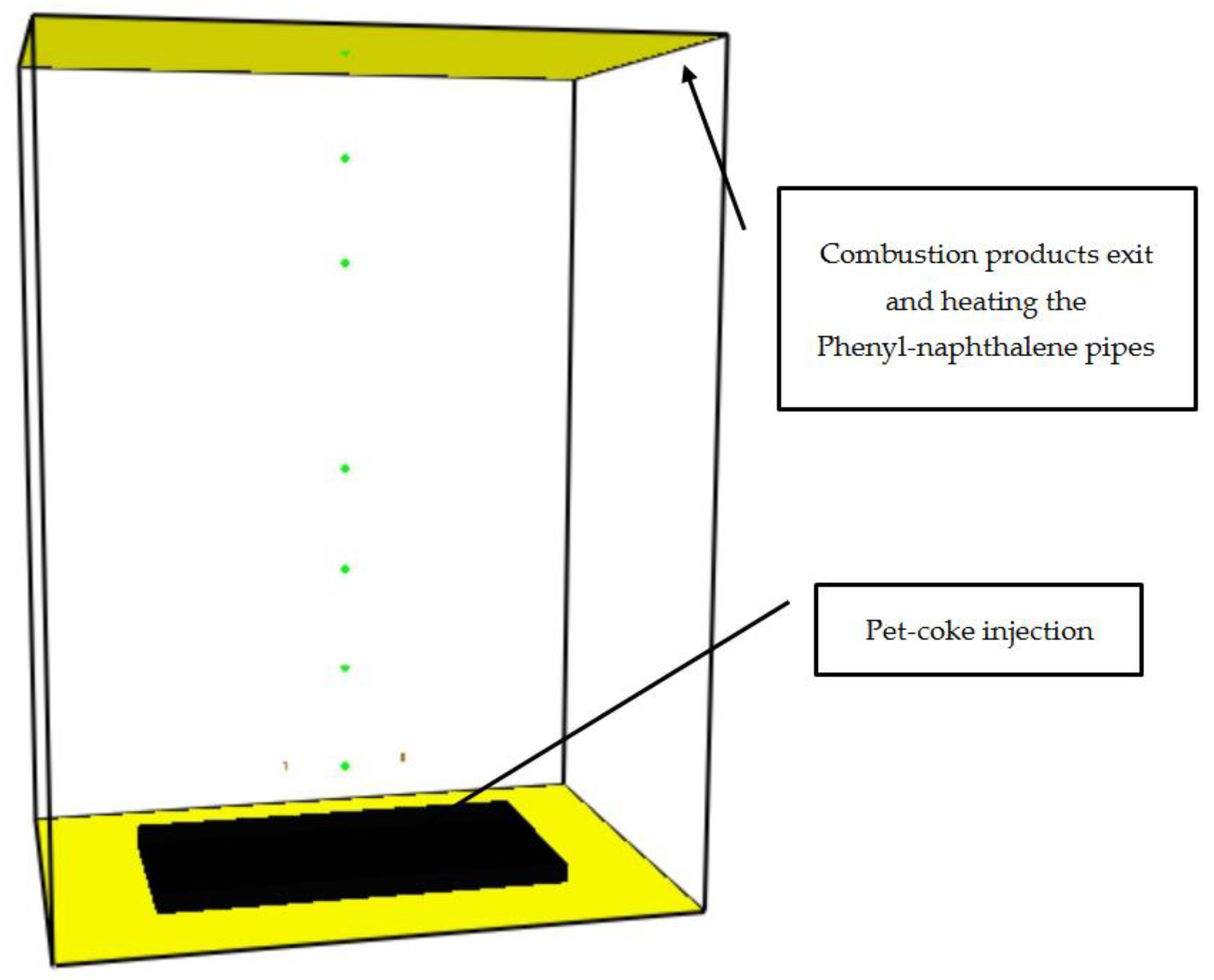
2.1. Fire Dynamic Simulation (FDS) Modeling of the Pet-Coke Burner
2.2. FDS Modelling of the Combustor
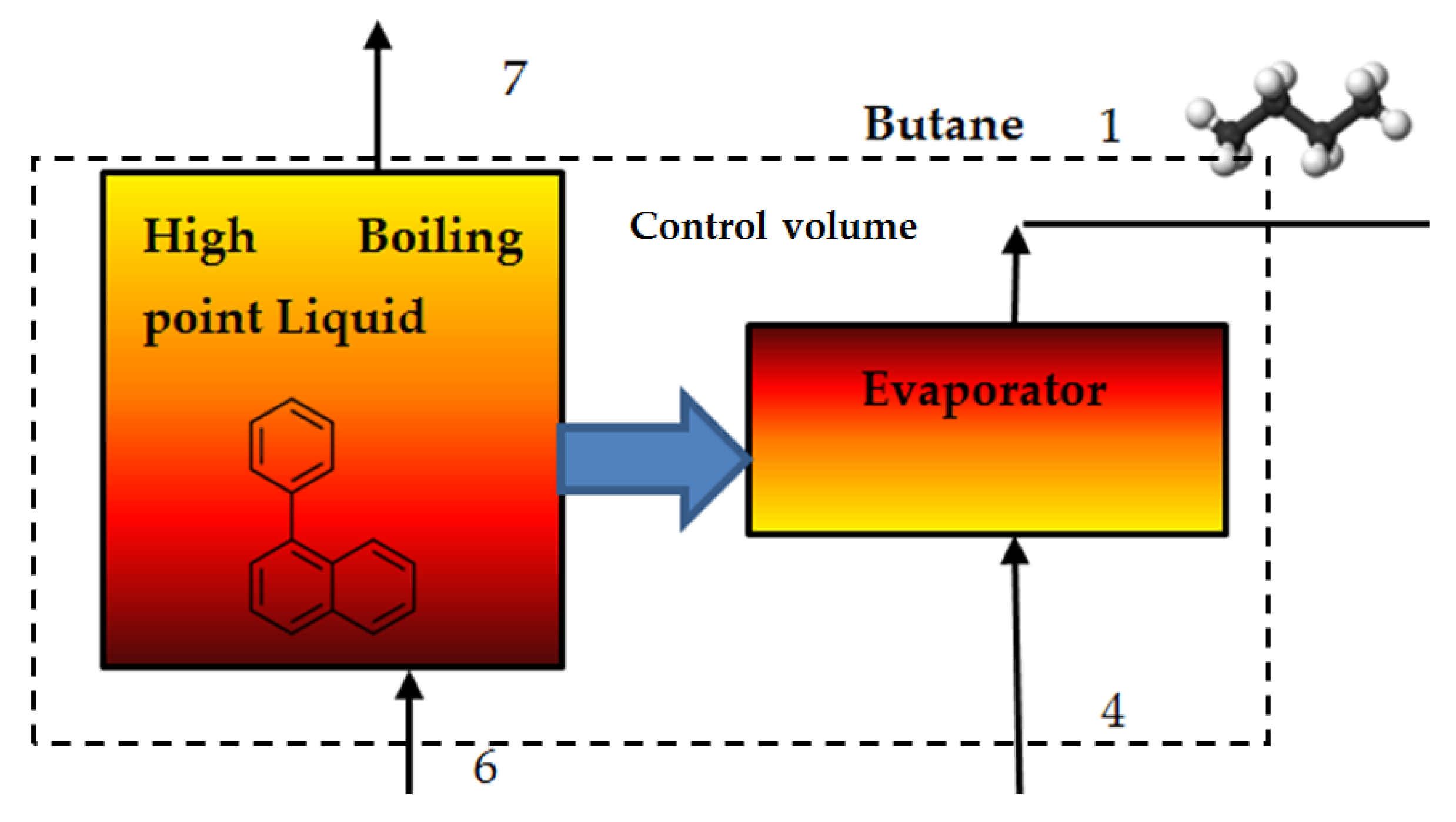
2.3. Thermodynamic Analysis of the Organic Rankine Cycle
2.4. Calculation of Net Entropy Change Rate of the O
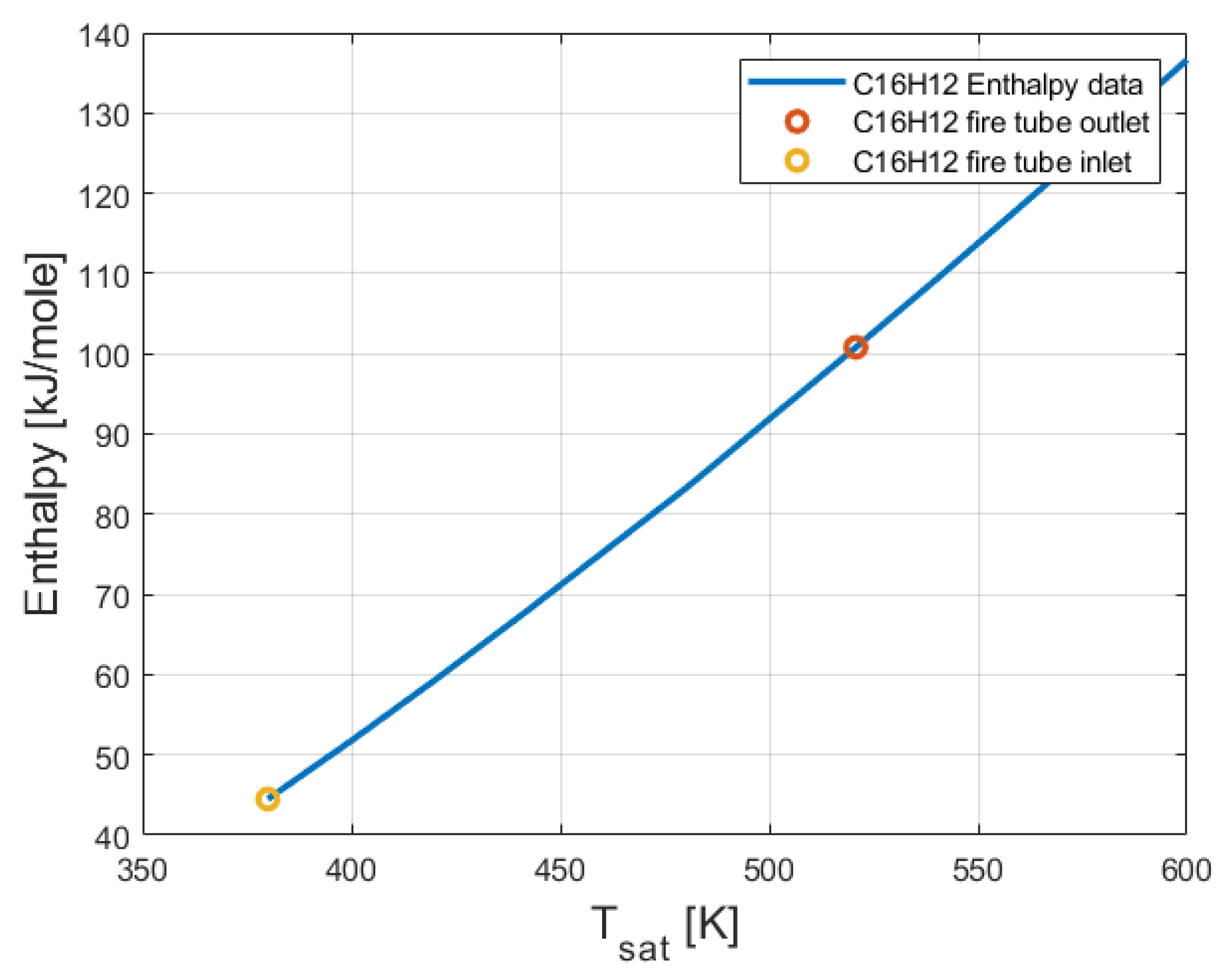
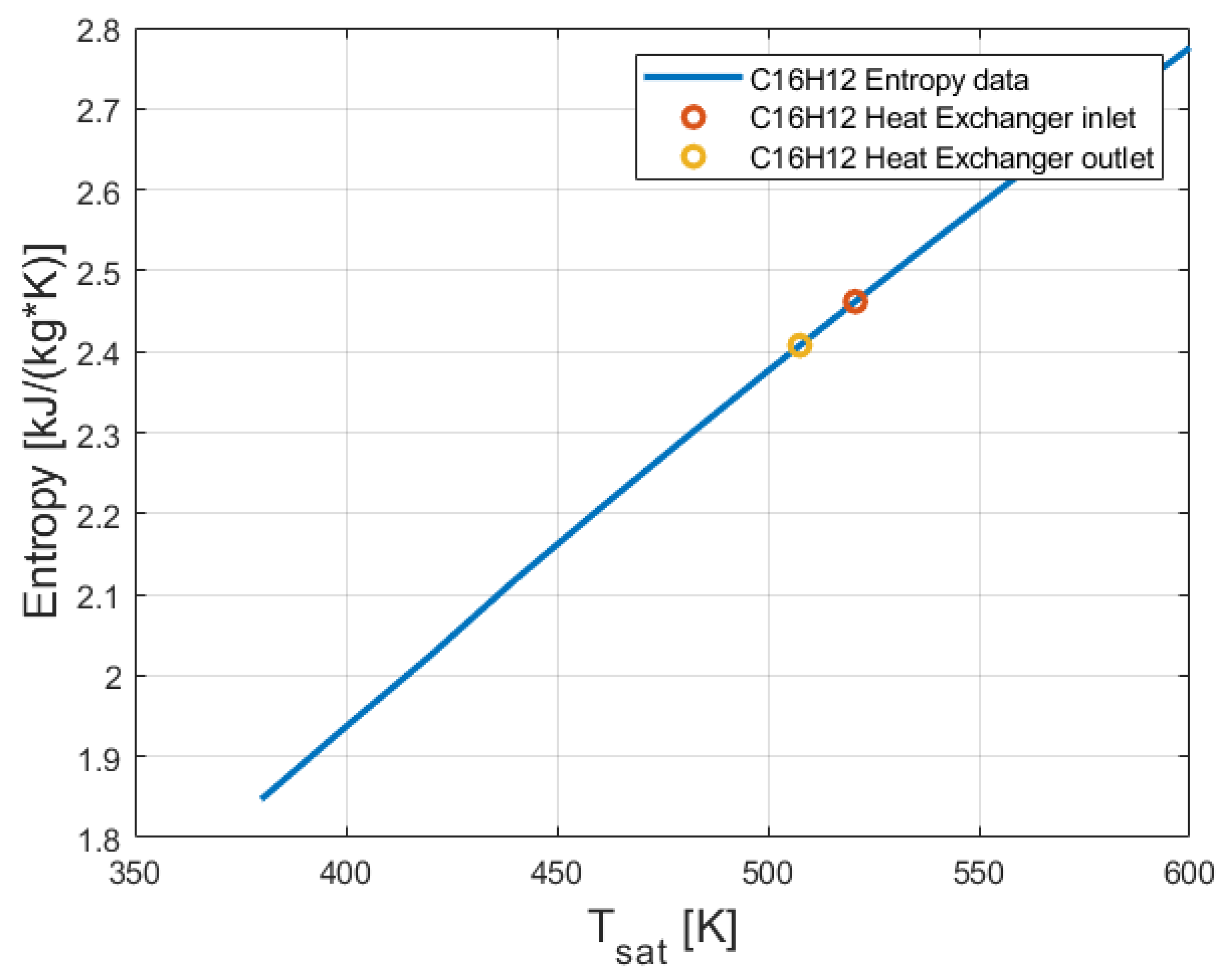
2.5. Thermo-Physical and Thermodynamic Properties of 2-Phenylnaphthalene (C16H12)
3. Results
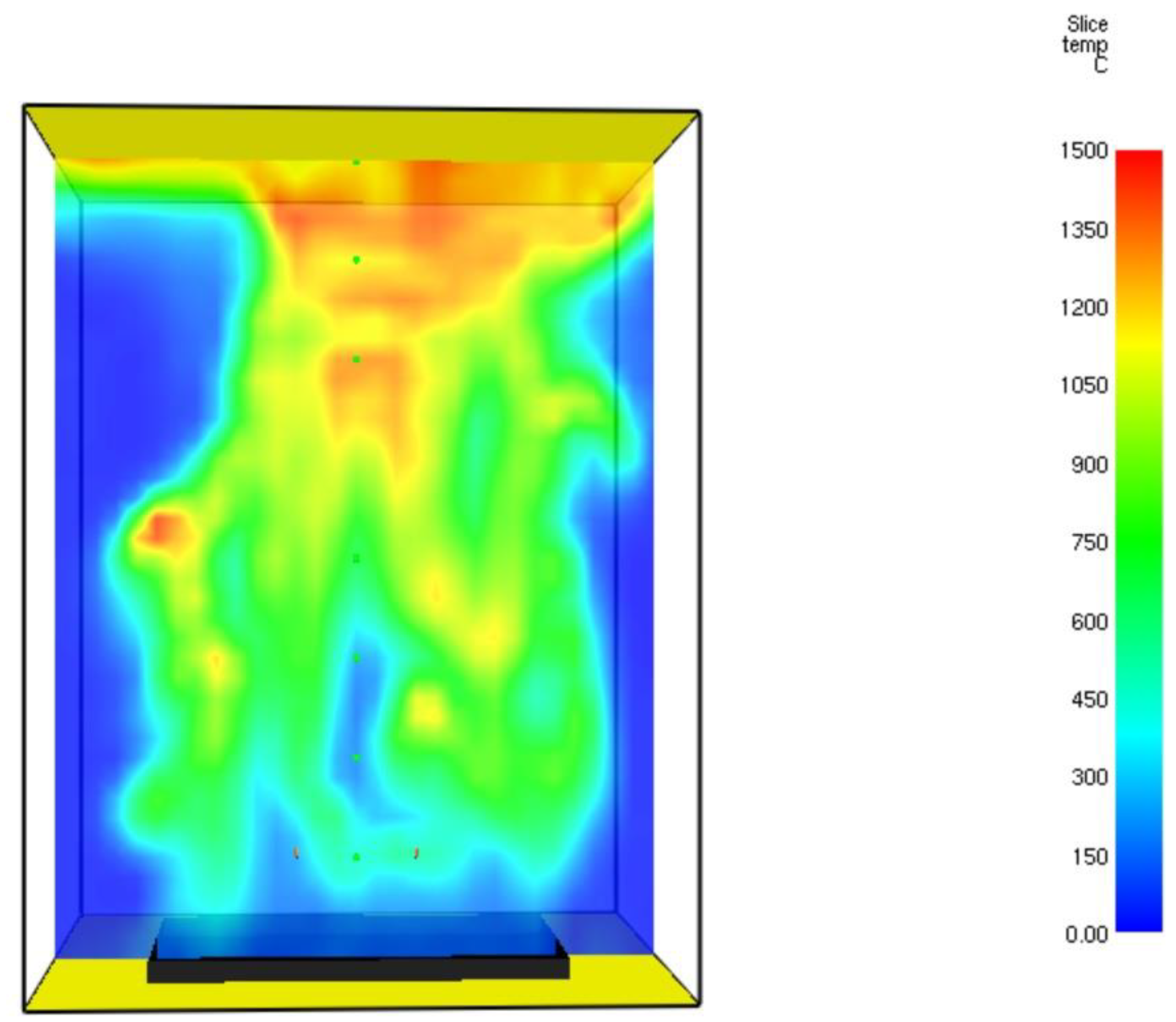
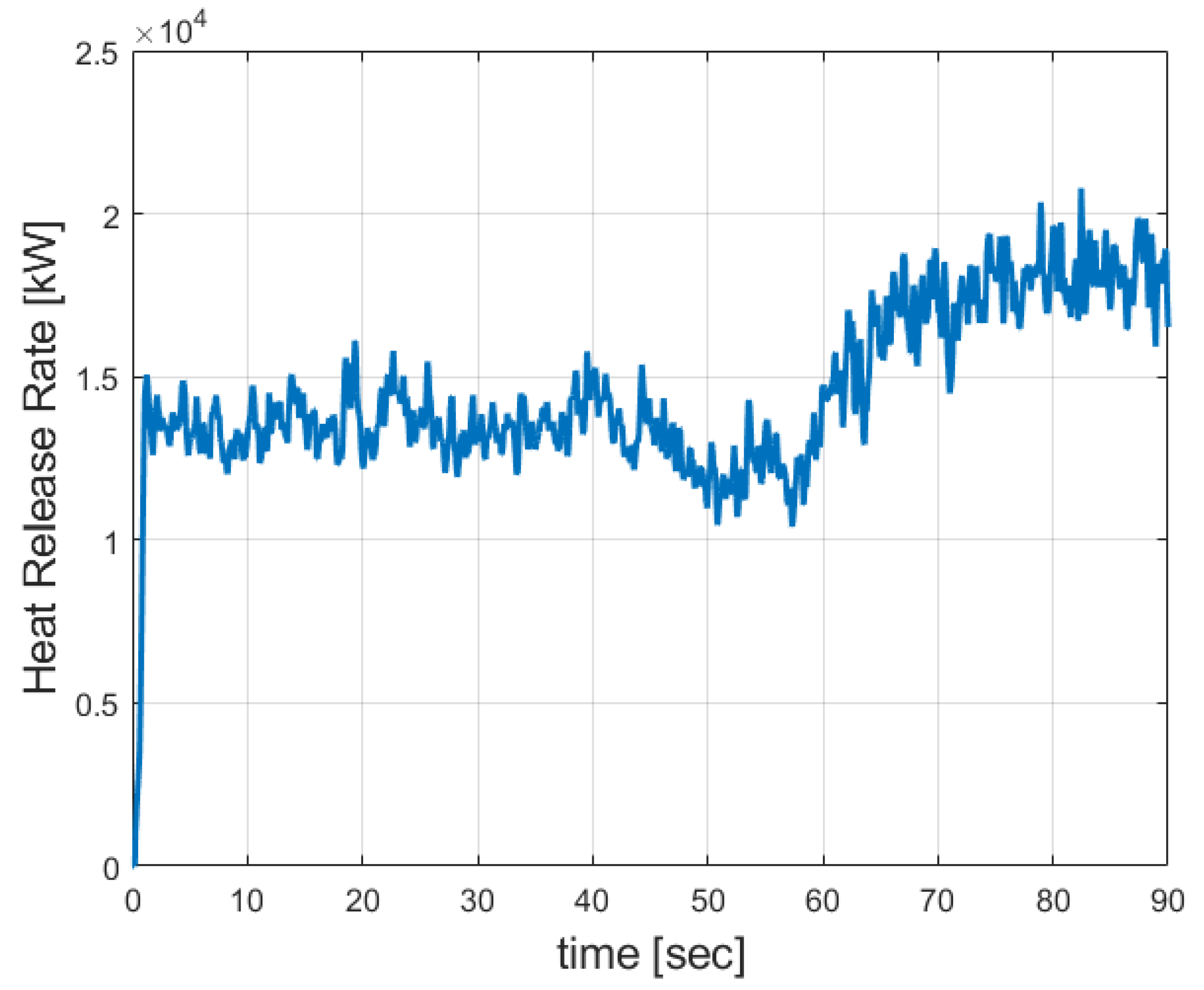
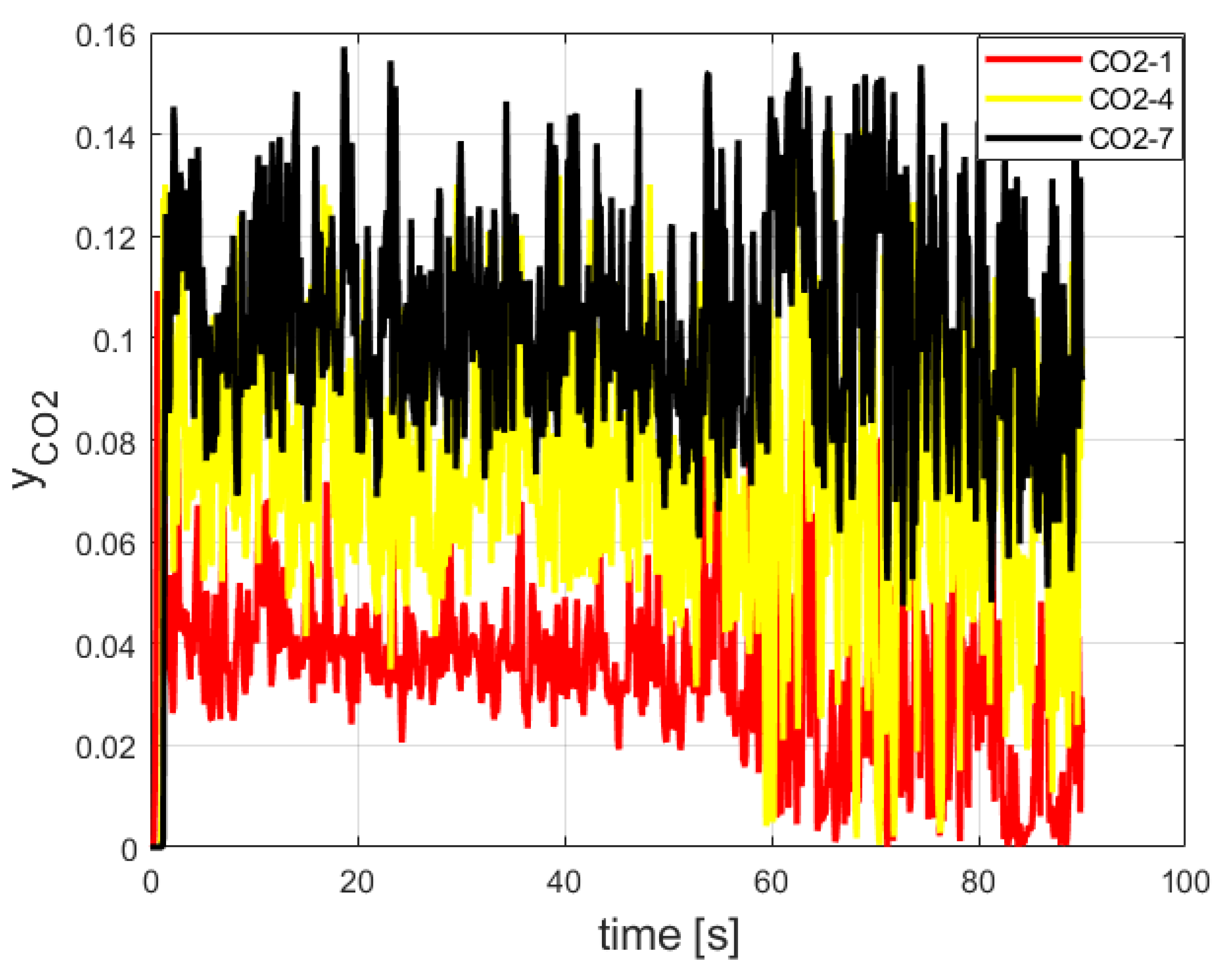
3.1. FDS Results for the Burner
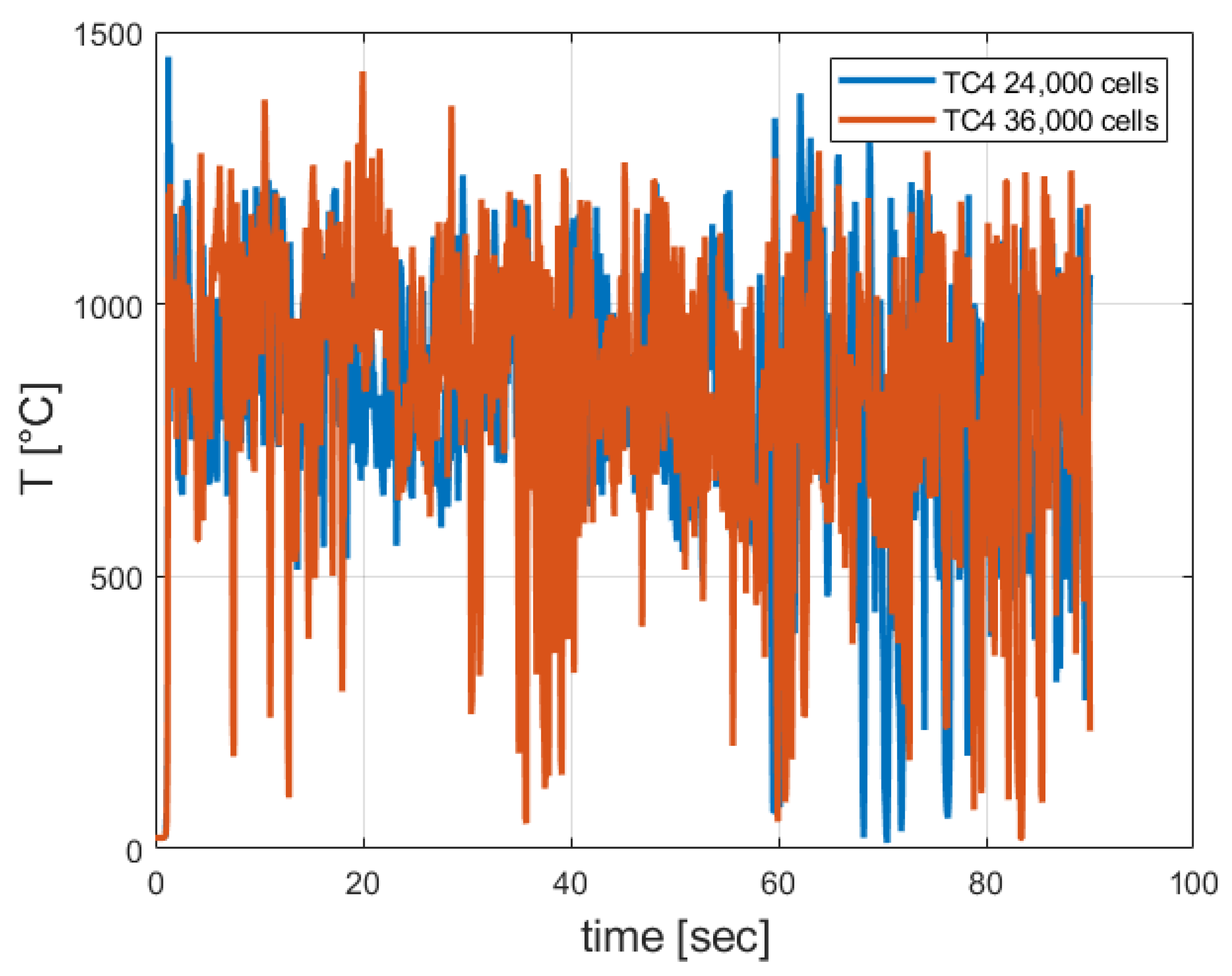
3.2. Grid Sensitivity Study Results
3.3. Thermodynamic Analysis Results of the Organic Rankine Cycle (ORC)
4. Conclusions
5. Discussion and Future Work
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFD | Computational Fluid Dynamics |
| CTL | Coal to Liquids |
| FDS | Fire Dynamics Simulation |
| F-T | Fischer Tropsch |
| HRR | Heat Release Rate |
| SSSF | Steady State Steady Flow |
| RTE | Radiation Transport Equation |
| gravity acceleration in [m/s2] | |
| specific entropy of the stream in [kJ/(kg K)] | |
| time in [s] | |
| velocity of the entering/leaving stream in [m/s] | |
| height of the stream in [m] | |
| Subscripts | |
| condenser | |
| control volume | |
| leaving | |
| Evaporator | |
| Heat Release Rate | |
| entering | |
| net | |
| pump | |
| turbine | |
| Greek letters | |
| thermal efficency of the Organic Rankine Cycle | |
| density in [kg/m3] | |
References
- Exergy, Organic Rankine Cycle Technology Can Drive Efficiency Improvements across the Energy Industry. Available online: https://www.nsenergybusiness.com/news/exergy-organic-rankine-cycle/ (accessed on 1 October 2022).
- Li, J. Structural Optimization and Experimental Investigation of the Organic Rankine Cycle for Solar Thermal Power Generation. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2015. [Google Scholar]
- Hamadeh, H.; Toor, S.Y.; Douglas, P.L.; Sarathy, S.M.; Dibble, R.W.; Croiset, E. Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies 2020, 13, 3463. [Google Scholar] [CrossRef]
- Shen, J.; Schmetz, E.; Stiegel, G.J.; Winslow, J.C.; Kornosky, R.M.; Madden, D.R.; Jain, S.C. Early Entrance Coproduction Plant—The Pathway to the Commercial CTL (Coal-to-Liquids) Fuels Production. In Studies in Surface Science and Catalysis; Davis, B.H., Occelli, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 163, pp. 315–325. ISSN 0167-2991. ISBN 9780444522214. [Google Scholar] [CrossRef]
- Méndez-Cruz, L.E.; Gutiérrez-Limón, M.Á.; Lugo-Méndez, H.; Lugo-Leyte, R.; Lopez-Arenas, T.; Sales-Cruz, M. Comparative Thermodynamic Analysis of the Performance of an Organic Rankine Cycle Using Different Working Fluids. Energies 2022, 15, 2588. [Google Scholar] [CrossRef]
- Fernández-Guillamón, A.; Molina-García, Á.; Vera-García, F.; Lmendros-Ibáñez, J.A. Organic Rankine Cycle Optimization Performance Analysis Based on Super-Heater Pressure: Comparison of Working Fluids. Energies 2021, 14, 2548. [Google Scholar] [CrossRef]
- Chechetkin, A.V. High Temperature Heat Carriers. In Pergamon Press Book; The Macmillam Company: New York, NY, USA, 1963. [Google Scholar]
- Shirvan, K.; Forest, E. Design of an Organic Simplified Nuclear Reactor. Nucl. Eng. Technol. 2016, 48, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, G.F. Chapter 7: Pool Boiling in Butterworth. In Two Phase Flow and Heat Transfer; Hewitt, G.F., Ed.; Oxford University Press: Oxford, UK, 1977. [Google Scholar]
- Davidy, A. CFD Design of Hydrogenation Reactor for Transformation of Levulinic Acid to γ-Valerolactone (GVL) by using High Boiling Point Organic Fluids. ChemEngineering 2019, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- McCabe, W.L.; Smith, J.C. Unit Operations of Chemical Engineering, 2nd ed.; MacGraw-Hill, Inc.: New York, NY, USA, 1967. [Google Scholar]
- McGrattan, K. Fire Dynamics Simulator (Version 5)—Technical Reference Guide Volume 1: Mathematical Model; NIST Special Publication 1018; National Institute of Standards and Technology U.S. Department of Commerce: Washington, DC, USA, 2010. [Google Scholar]
- McGrattan, K.; Forney, G.P. Fire Dynamics Simulator (Version 5)—User’s Guide; NIST Special Publication 1019; National Institute of Standards and Technology U.S. Department of Commerce: Washington, DC, USA, USA, 2010. [Google Scholar]
- McGrattan, K. Numerical Simulation of the Caldecott Tunnel Fire, April 1982; NISTIR 7231; National Institute of Standards and Technology U.S. Department of Commerce: Washington, DC, USA, 2005. [Google Scholar]
- Davidy, A. CFD Simulation of Forced Recirculating Fired Heated Reboilers. Processes 2020, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Davidy, A. Multiphysics Design of Pet-Coke Burner and Hydrogen Production by Applying Methane Steam Reforming System. Clean Technol. 2021, 3, 260–287. [Google Scholar] [CrossRef]
- Magee, J.S. Fluid Catalytic Cracking: Science and Technology, Studies in Surface Science and Catalysis. In Studies in Surface Science and Catalysis; Delmon, B., Yates, J.T., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1993; Volume 76. [Google Scholar]
- Pedersen, M.N.; Nielsen, M.; Clausen, S.; Jensen, P.A.; Jensen, L.S.; Johansen, K.D. Imaging of Flames in Cement Kilns to Study the Influence of Different Fuel Types. Energy Fuels 2017, 31, 11424–11438. [Google Scholar] [CrossRef] [Green Version]
- Van Wylen, G.J.; Sonntag, R.E. Fundamentals of Classical Thermodynamics, 3rd ed.; John Wiley and Sons Inc.: Toronto, ON, Canada, 1985. [Google Scholar]
- Thermoptim Website. Available online: https://direns.mines-paristech.fr/Sites/Thopt/en/co/applet-calcualteur.html (accessed on 29 July 2022).
- McFarlane, J.; Luo, H.; Garland, M.; Steele, W.V. Evaluation of Phenyl-naphthalenes as Heat Transfer Fluids for High Temperature Energy Applications. Sep. Sci. Technol. 2010, 45, 1908–1920. [Google Scholar] [CrossRef]
- Commandre, J.L.; Salvador, S. Lack of Correlation between the Properties of a Petroleum Coke and Its Behavior during Combustion. Fuel Processing Technology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 86, pp. 795–808. [Google Scholar]
- Sandmo, T. The Norwegian Emission Inventory, Documentation of Methodologies for Estimating Emissions of Greenhouse Gases and Long-Range Transboundary Air Pollutant; Statistics Norway/Department of Economics, Energy and the Environment Statistics: Oslo, Norway, 2009. [Google Scholar]
- Castelli, A.F.; Elsido, C.; Scaccabarozzi, R.; Nord Lars, O.; Martelli, E. Optimization of Organic Rankine Cycles for Waste Heat Recovery from Aluminum Production Plants. Front. Energy Res. 2019, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Hodnebrog, Ø.; Dalsøren, S.B.; Myhre, G. Lifetimes, direct and indirect radiative forcing, and global warming potentials of ethane (C2H6), propane (C3H8), and butane (C4H10). Atmos. Sci. Lett. 2018, 19, e804. [Google Scholar] [CrossRef]
- Bronicki, L.Y. History of Organic Rankine Cycle systems, in Organic Rankine Cycle (ORC) Power Systems Technologies and Applications; Macchi, E., Astolfi, M., Eds.; Woodhead Publishing Series in Energy: Number 107; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Butane#section=Viscosity (accessed on 28 July 2022).
- Lewis, R.J., Sr. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; p. 190. [Google Scholar]
- Bao, J. Organic Rankine Cycle for Recovery of Liquefied Natural Gas (LNG) Cold Energy. In Organic Rankine Cycle Technology for Heat Recovery; Wang, E., Ed.; Intech-Open: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Grisolia, G.; Fino, D.; Lucia, U. Thermodynamic optimization of the biofuel production based on mutualism. Energy Rep. 2020, 6, 1561–1571. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G. Biofuels Analysis Based on the THDI Indicator of Sustainability. Front. Energy Res. 2021, 9, 794682. [Google Scholar] [CrossRef]





| Material Property | Value |
|---|---|
| ρ | 335 (kg/m3) |
| Cp | 2364 (J/(kg·°C)) |
| k | 0.083 (w/(m·°C)) |
| η | 0.00011 (Pa·s)) |
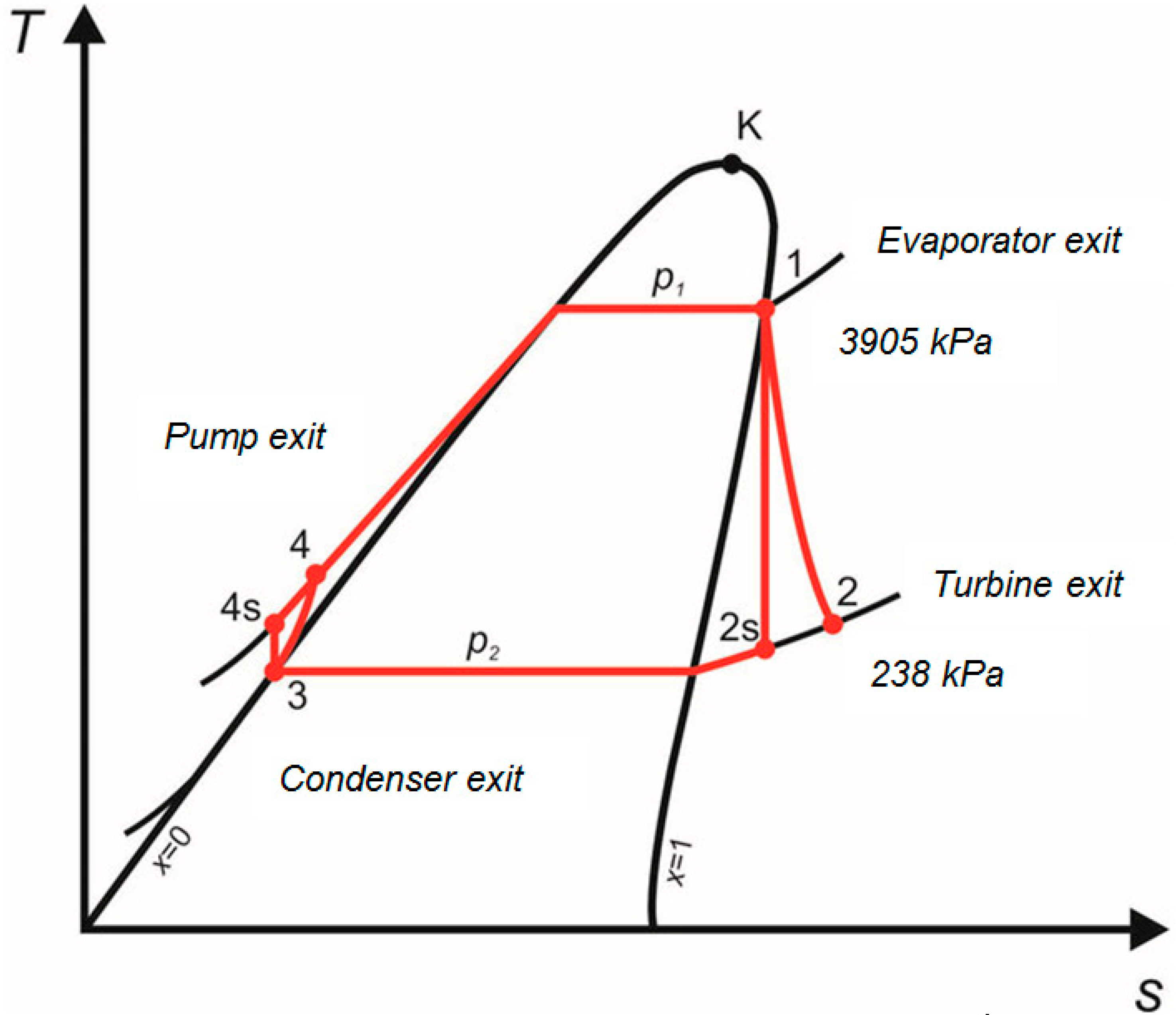
| Point | Pressure [kPa] | Temperature [°C] | Enthalpy, h [kJ/kg] | Entropy, s [kJ/(kg K)] |
|---|---|---|---|---|
| 1 | 3905 | 164.2 | 801.15 | 2.618 |
| 2 | 238 | 57.5 | 686.4 | 2.632 |
| 3 | 238 | 0.0 | 199.8 | 0.999 |
| 4 | 3905 | 0.5 | 207.45 | 1.004 |
| Power/Heat | Value [kW] |
|---|---|
| 18,072 | |
| −14,811 | |
| 3260 | |
| 3493 | |
| −232.3 | |
| 3260 |
| Component | [kW/K] | |
|---|---|---|
| 0.426 | 0.127 | |
| 4.515 | 1.346 | |
| 0.152 | 0.045 | |
| 46.43 | 13.800 | |
| 24.34 | 7.256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidy, A. Large Eddy Simulation and Thermodynamic Design of the Organic Rankine Cycle Based on Butane Working Fluid and the High-Boiling-Point Phenyl Naphthalene Liquid Heating System. Entropy 2022, 24, 1461. https://doi.org/10.3390/e24101461
Davidy A. Large Eddy Simulation and Thermodynamic Design of the Organic Rankine Cycle Based on Butane Working Fluid and the High-Boiling-Point Phenyl Naphthalene Liquid Heating System. Entropy. 2022; 24(10):1461. https://doi.org/10.3390/e24101461
Chicago/Turabian StyleDavidy, Alon. 2022. "Large Eddy Simulation and Thermodynamic Design of the Organic Rankine Cycle Based on Butane Working Fluid and the High-Boiling-Point Phenyl Naphthalene Liquid Heating System" Entropy 24, no. 10: 1461. https://doi.org/10.3390/e24101461








