Application of a Speedy Modified Entropy Method in Assessing the Complexity of Baroreflex Sensitivity for Age-Controlled Healthy and Diabetic Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Experimental Procedure
2.1.1. Study Population and Grouping
2.1.2. Experimental Procedure
2.2. Study Protocol
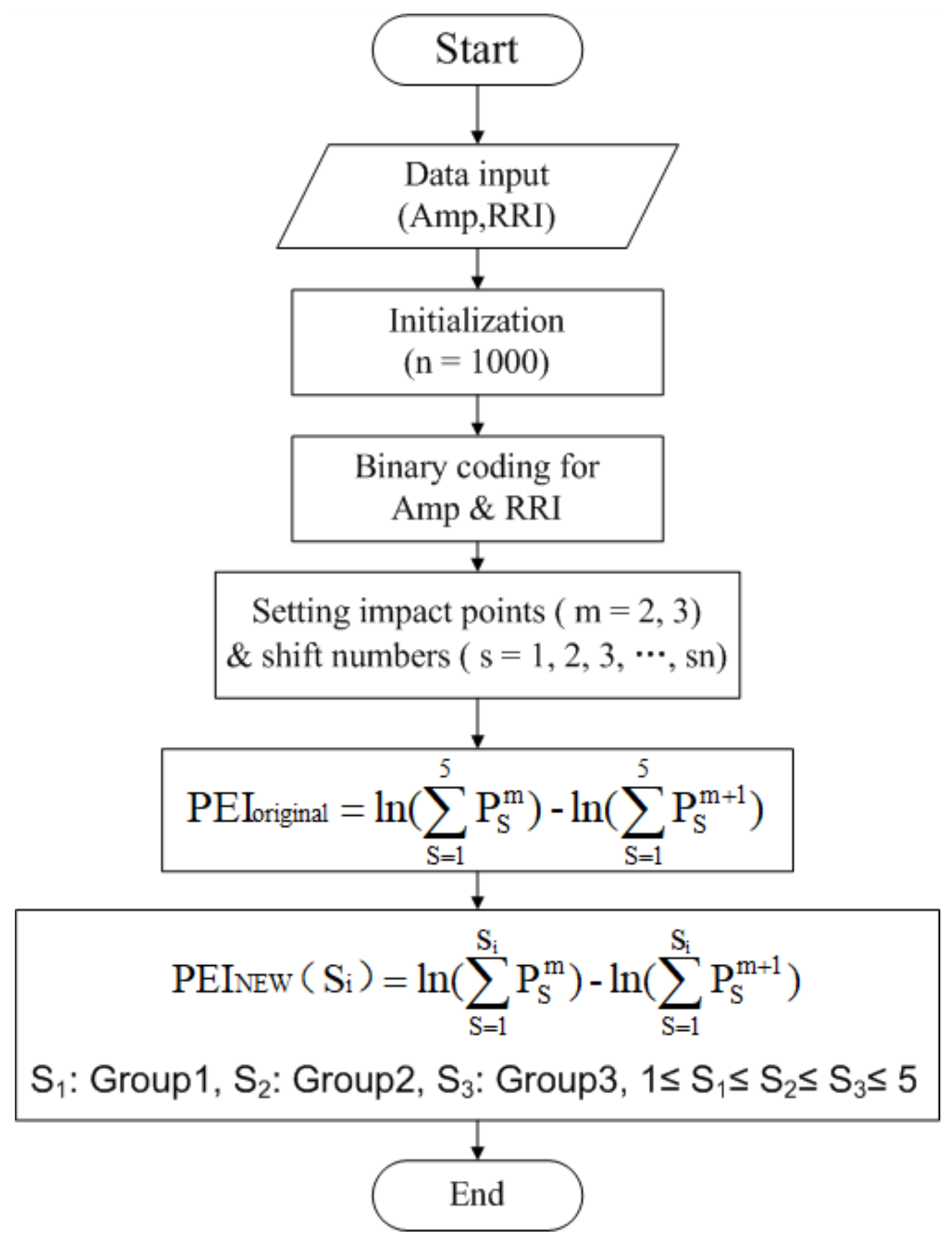
2.3. A Speedy Modified Entropy Method for Assessing the Complexity of Baroreflex Sensitivity
2.3.1. Percussion Entropy Index, PEIoriginal
- (1)
- Taking BRS regulation into account, the binary sequence transformations for Amp and RRI were computed:
- (2)
- The n − m + 1 vectors of patterns for BAmp and BRRI, each of size m, were defined, and these were composed as follows:BAmp(i) = {ai, ai+1,…, ai+m-1}, 1 ≤ i ≤ n − m + 1For s = 1–5 (i.e., shift numbers), the series BRRI,BRRI(i+s) = {ri+s, ri+s +1,…, ri+s+m-1}, 1 ≤ i ≤ n − m + 1, s = 1 to 5.
- (3)
- The percussion rate (i.e., the similarity in the pattern of fluctuation) for BAmp(i) and BRRI(i+s) was counted with the given m.Then, the total match number of BAmp(i) and BRRI(i+s) was counted with the same pattern (i.e., the percussion number) and divided by the total number of vectors of patterns (n – m – s + 1) to obtain the percussion rate, which was expressed as
- (4)
- The logarithm of the sum of percussion rates (Pms) from shift numbers 1–5 (i.e., s = 1, 2, 3, 4, 5) gave
- (5)
- The embedded dimension was increased to (m + 1), and (9) and (10) changed to
- (6)
2.3.2. A Speedy Modified Percussion Entropy Index, PEINEW
- Assuming S1 = 1, calculate the Pearson correlation coefficients (r) of PEINEW (1) and PEINEW (2); PEINEW (1) and PEINEW (3); PEINEW (1) and PEINEW (4); and PEINEW (1) and PEINEW (5).
- If {r > 0.8, and is statistically significant, (p < 0.05)} and {PEINEW (1) and PEINEW (2) show good agreement}, then stop (S1 = 1). Subsequently, go to S2 selection; otherwise, go to the next step.
- Assuming S1 = 2, calculate the Pearson correlation coefficients (r) of PEINEW (2) and PEINEW (3); PEINEW (2) and PEINEW (4); and PEINEW (2) and PEINEW (5).
- If {r > 0.8 and is statistically significant, (p < 0.05)} and {PEINEW (2) and PEINEW (3) show good agreement}, then stop (S1 = 2). Subsequently, go to S2 selection; otherwise, go to the next step.
- Assuming S1 = 3, calculate the Pearson correlation coefficients (r) of PEINEW (3) and PEINEW (4) and PEINEW (3) and PEINEW (5).
- If {r > 0.8 and is statistically significant, (p < 0.05)} and {PEINEW (3) and PEINEW (4) show good agreement}, then stop (S1 = 3). Subsequently, go to S2 selection; otherwise go to the next step.
- Assuming S1 = 4, calculate the Pearson correlation coefficient (r) of PEINEW (4) and PEINEW (5).
- If {r > 0.8 and is statistically significant, (p < 0.05)} and {PEINEW (4) and PEINEW (5) show good agreement}, then stop (S1 = 4). Subsequently, go to S2 selection; otherwise, stop.
2.4. Computation Times for Comparison
2.5. Statistical Analysis
3. Results
3.1. Optimal Prolongation between the Amplitude Series and RRI Series for the Three Groups
3.1.1. A Simple Way to Estimate the Delay between Amp and RRI
- S1 Selection for Group 1. As shown in Table 2, two PEINEW sequences in (16) were computed from cases A–D for Group 1, followed by the Pearson correlation calculation for the two sequences. For example, we obtained two time series, PEINEW(1) and PEINEW(2), in case A for Group 1 subjects, which were very highly correlated (r = 0.91) and statistically significant (p = 0.01). Then, the optimal shift number for Group 1 was expressed as 1 (i.e., S1 = 1).
- S2 Selection for Group 2. For Group 2 subjects, as in Step 1, we obtained two time series, PEINEW(3) and PEINEW(4), in case H for Group 2 subjects, which were very highly correlated (r = 0.84 > 0.8) and statistically significant (p < 0.00) (Table 2). After checking the Bland–Altman plot (Figure 2b), the optimal shift number for Group 2 was expressed as 3 (i.e., S2 = 3).
- S3 Selection for Group 3. For Group 3 subjects, as in Step 1, we obtained two time series, PEINEW(4) and PEINEW(5), in case J for Group 3 subjects, which were very highly correlated (r = 0.87 > 0.8) and statistically significant (p < 0.00) (Table 2). After checking the Bland–Altman plot (Figure 2c), the optimal shift number for Group 3 was expressed as 4 (i.e., S3 = 4).
3.1.2. Reproducibility Analysis for PEINEW and PEIorginal for All Subjects
3.1.3. Correlation between PEINEW and PEIorginal for the Three Groups
3.2. Comparison among MCAE, PEIoriginal, and PEINEW for Autonomic Function Assessment in All Testing Subjects
3.3. Correlations of Demographic, Anthropometric, Hemodynamic, and Serum Biochemical Data with MCAE, PEIoriginal, and PEINEW
3.4. Computation Time for MCAE, PEIoriginal, and PEINEW in All Testing Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Debono, M.; Cachia, E. The impact of cardiovascular autonomic neuropathy in diabetes: Is it associated with left ventricular dysfunction? Auton. Neurosci. Basic Clin. 2007, 132, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E. Heart rate variability- standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Pozza, R.D.; Bechtold, S.; Bonfig, W.; Putzker, S.; Kozlik-Feldmann, R.; Schwarz, H.P.; Netz, H. Impaired short-term blood pressure regulation and autonomic dysbalance in children with type 1 diabetes mellitus. Diabetologia 2007, 50, 2417–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosengard-Barlund, M.; Bernardi, L.; Fagerudd, J.; Mantysaari, M.; Bjorkesten, G.G.A.; Lindholm, H.; Forsblom, C.; Waden, J.; Groop, P.H. Early autonomic dysfunction in type 1 diabetes: A reversible disorder? Diabetologia 2009, 52, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Swenne, C.A. Baroreflex sensitivity: Mechanisms and measurement. Neth. Heart J. 2013, 21, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Syamsunder, A.N.; Pal, P.; Pal, G.K.; Kamalanathan, C.S.; Parija, S.C.; Nanda, N.; Sirisha, A. Decreased baroreflex sensitivity is linked to the atherogenic index, retrograde inflammation, and oxidative stress in subclinical hypothyroidism. Endocr. Res. 2017, 42, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rosengard-Barlund, M.; Bernardi, L.; Holmqvist, J.; Debarbieri, G.; Mantysaari, M.; Bjorkesten, C.G.A.; Forsblom, C.; Groop, P.H. Deep breathing improves blunted baroreflex sensitivity even after 30 years of type 1 diabetes. Diabetologia 2011, 54, 1862–1870. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Singer, W.; Gehrking, T.L.; Sletten, D.M.; Schmelzer, J.D.; Low, P.A. Comparison of baroreflex sensitivity with a fall and rise in blood pressure induced by the Valsalva maneuver. Clin. Sci. 2014, 127, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef]
- Wu, H.T.; Lee, K.W.; Pan, W.Y.; Liu, A.B.; Sun, C.K. Difference in bilateral digital volume pulse as a novel non-invasive approach to assessing arteriosclerosis in aged and diabetic subjects: A preliminary study. Diabetes Vasc. Dis. Res. 2017, 14, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.; Jun, S.; Um, D.; Oh, J.; Yoon, H. Non-invasive estimation of systolic blood pressure and diastolic blood pressure using photoplethysmograph components. Yonsei Med. J. 2010, 51, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 2016, 7, 3007–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Min, S.D. Feasibility study for the non-invasive blood pressure estimation based on ppg morphology: Normotensive subject study. Biomed. Eng. Online 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Lee, C.Y.; Liu, C.C.; Liu, A.B. Multiscale cross-approximate entropy analysis as a measurement of complexity between ECG R-R interval and PPG pulse amplitude series among the normal and diabetic subjects. Comput. Math. Methods Med. 2013, 2013, 231762. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.C.; Xiao, M.X.; Ta, N.; Wu, H.T.; Sun, C.K. Assessment of diabetic autonomic nervous dysfunction with a novel percussion entropy approach. Complexity 2019, 2019, 6469853. [Google Scholar] [CrossRef]
- Martinez-Garcia, P.; Lerma, C.; Infante, O. Baroreflex sensitivity estimation by the sequence method with delayed signals. Clin. Auton. Res. 2012, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.K.; Hsiai, T.K.; Chiao, J.-C.; Cao, H. Cuff-less and continuous blood pressure monitoring: A methodological review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef]
- Dutoit, A.P.; Hart, E.C.; Charkoudian, N.; Wallin, B.G.; Curry, T.B.; Joyner, M.J. Cardiac baroreflex sensitivity is not correlated to sympathetic baroreflex sensitivity within healthy, young humans. Hypertension 2010, 56, 6. [Google Scholar] [CrossRef]
- Miyai, N.; Arita, M.; Morioka, I.; Miyashita, K.; Nishio, I.; Takeda, S. Exercise BP response in subjects with high-normal BP. J. Am. Coll. Cardiol. 2000, 36, 1626–1631. [Google Scholar] [CrossRef]
- Huggett, R.J.; Scott, E.M.; Gilbey, S.G.; Stoker, J.B.; Mackintosh, A.F.; Mary, D.A.S.G. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003, 108, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Casellini, C.; Parson, H.K.; Colberg, S.R.; Nevoret, M.L. Cardiac autonomic neuropathy in diabetes: A predictor of cardiometabolic events. Front. Neurosci. 2018, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Nikhar, S.; Ramachandran, G. Autonomic disturbances in diabetes: Assessment and anaesthetic implications. Indian J. Anaesth. 2018, 62, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.F.D.; Christofaro, D.G.D.; Bernardo, A.F.B.; Vanderlei, F.M.; Vanderlei, L.C.M. Sensitivity, specificity and predictive value of heart rate variability indices in type 1 diabetes mellitus. Arq. Bras. Cardiol. 2017, 108, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.M.; Giacon, T.R.; Pacagnelli, F.L.; Barbosa, M.P.C.R.; Valenti, V.E.; Vanderlei, L.C.M.; Vanderlei, L.C. Dynamics of heart rate variability analysed through nonlinear and linear dynamics is already impaired in young type 1 diabetic subjects. Cardiol. Young 2016, 26, 1383–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javorka, M.; Lazarova, Z.; Tonhajzerova, I.; Turianikova, Z.; Honzikova, N.; Fiser, B.; Javorka, K.; Baumert, M. Baroreflex analysis in diabetes mellitus: Linear and nonlinear approaches. Med. Biol. Eng. Comput. 2011, 49, 279. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef]
- Wu, H.T.; Lee, C.H.; Liu, A.B.; Chung, W.S.; Tang, C.J.; Sun, C.K.; Yip, H.K. Arterial stiffness using radial arterial waveforms measured at the wrist as an indicator of diabetic control in the elderly. IEEE Trans. Biomed. Eng. 2011, 58, 243–252. [Google Scholar]
- Wu, H.T.; Hsu, P.C.; Lin, C.F.; Wang, H.J.; Sun, C.K.; Liu, A.B.; Lo, M.T.; Tang, C.J. Multiscale entropy analysis of pulse wave velocity for assessing atherosclerosis in the aged and diabetic. IEEE Trans. Biomed. Eng. 2011, 58, 2978–2981. [Google Scholar]
- Draghici, A.E.; Taylor, J.A. The physiological basis and measurement of heart rate variability in humans. J. Physiol. Anthropol. 2016, 35, 22. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Parati, G.; Radaelli, A.; Castiglioni, P. Baroreflex contribution to blood pressure and heart rate oscillations: Time scales, time-variant characteristics and nonlinearities. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 1301–1318. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronda, E.; Francis, D.; Zannad, F.; Hamm, C.; Brugada, J.; Vanoli, E. Baroreflex Activation Therapy: A New Approach to The Management of Advanced Heart Failure with Reduced Ejection Fraction. J. Cardiovasc. Med. 2017, 18, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.; Eddie, D. Dynamic Processes in Regulation and Some Implications for Biofeedback and Biobehavioral Interventions. Appl. Psychophysiol. Biofeedback 2013, 38, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.; Monbaron, D.; Parati, G.; Perret, S.; Haesler, E.; Danzeisen, C.; Hayoz, D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 2005, 46, 162–167. [Google Scholar] [CrossRef]
- Xiao, M.X.; Wei, H.C.; Xu, Y.J.; Wu, H.T.; Sun, C.K. Combination of R-R Interval and Crest time in assessing complexity using multiscale cross-approximate entropy in normal and diabetic subjects. Entropy 2018, 20, 497. [Google Scholar] [CrossRef]


| Parameters | Group 1 Number: 42 Female/Male (24/18) | Group 2 Number: 38 Female/Male (17/21) | Group 3 Number: 35 Female/Male (12/23) |
|---|---|---|---|
| Age, years | 56.73 ± 3.80 | 60.05 ± 8.29 | 58.08 ± 11.33 |
| Body height, cm | 163.50 ± 8.33 | 163.59 ± 7.98 | 162.41 ± 5.18 |
| Body weight, kg | 65.00 ± 13.80 | 71.60 ± 11.82 | 79.60 ± 16.22 |
| WC, cm | 81.75 ± 11.80 | 94.35 ± 9.75 ** | 101.01 ± 13.49 †† |
| BMI, kg/m2 | 24.16 ± 4.07 | 26.53 ± 2.82 * | 29.81 ± 6.15 |
| SBP, mmHg | 116.46 ± 15.59 | 125.66 ± 18.02 | 125.69 ± 10.19 |
| DBP, mmHg | 73.69 ± 9.73 | 75.06 ± 12.36 | 76.35 ± 4.26 |
| PP, mmHg | 42.40 ± 10.70 | 51.55 ± 11.88 | 50.30 ± 12.08 |
| HDL, mg/dL LDL, mg/dL | 53.21 ± 20.80 122.35 ± 29.50 | 44.04 ± 9.89 94.36 ± 21.90 | 40.50 ± 9.62 118.10 ± 28.91 |
| Cholesterol, mg/dL Triglyceride, mg/dL | 192.45 ± 40.00 98.06 ± 85.36 | 170.81 ± 31.05 112.92 ± 39.92 | 199.10 ± 34.62 185.89 ± 74.90 |
| HbA1c, % | 5.69 ± 0.37 | 6.93 ± 0.39 ** | 9.25 ± 1.60 †† |
| FBS, mg/dL | 93.99 ± 10.65 | 127.45 ± 25.70 ** | 176.91 ± 68.51 †† |
| Case | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| A | 0.91 | 0.01 | 0.13 | 0.45 | 0.47 | 0.01 |
| B | 0.10 | 0.55 | 0.06 | 0.73 | 0.22 | 0.25 |
| C | −0.36 | 0.02 | 0.21 | 0.23 | 0.05 | 0.81 |
| D | −0.14 | 0.39 | −0.03 | 0.88 | 0.13 | 0.47 |
| E | - | - | 0.78 | 0.00 | 0.76 | 0.00 |
| F | - | - | 0.36 | 0.03 | 0.34 | 0.06 |
| G | - | - | 0.45 | 0.01 | 0.37 | 0.04 |
| H | - | - | 0.84 | 0.00 | 0.50 | 0.01 |
| I | - | - | - | - | 0.41 | 0.02 |
| J | - | - | - | - | 0.87 | 0.00 |
| Parameters | Group 1 (N = 42) | Group 2 (N = 38) | Group 3 (N = 35) |
|---|---|---|---|
| MCAE | 0.83 ± 0.08 | 0.74 ± 0.09 * | 0.75 ± 0.05 |
| PEIoriginal | 0.73 ± 0.04 | 0.63 ± 0.07 ** | 0.56 ± 0.09 † |
| PEINEW | 0.82 ± 0.04 | 0.65 ± 0.01 ** | 0.58 ± 0.01 †† |
| PEINEW | PEIoriginal | MCAE | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age (years) | 0.32 | 0.24 | 0.07 | 0.49 | 0.08 | 0.46 |
| BH (cm) | 0.01 | 0.90 | 0.16 | 0.09 | 0.19 | 0.08 |
| BW (kg) | –0.18 | 0.06 | –0.33 | 0.02 | 0.18 | 0.11 |
| WC(cm) | –0.25 | 0.01 | –0.42 | 0.00 | 0.00 | 0.98 |
| BMI (kg/m2) | –0.20 | 0.04 | –0.25 | 0.01 | 0.08 | 0.49 |
| SBP (mmHg) | –0.04 | 0.67 | –0.01 | 0.89 | 0.16 | 0.16 |
| DBP (mmHg) | –0.03 | 0.76 | –0.04 | 0.69 | 0.19 | 0.91 |
| PP (mmHg) | –0.04 | 0.72 | 0.01 | 0.90 | 0.09 | 0.45 |
| HDL (mg/dL) | 0.09 | 0.35 | 0.13 | 0.20 | 0.02 | 0.84 |
| LDL (mg/dL) | –0.15 | 0.14 | –0.20 | 0.04 | -0.16 | 0.18 |
| Cholesterol (mg/dL) | 0.10 | 0.33 | –0.09 | 0.37 | 0.17 | 0.08 |
| Triglyceride (mg/dL) | –0.27 | 0.01 | –0.31 | 0.00 | –0.21 | 0.00 |
| HbA1c (%) | –0.45 | 0.00 | –0.57 | 0.00 | –0.16 | 0.18 |
| FBS (mg/dL) | –0.29 | 0.00 | –0.53 | 0.00 | –0.73 | 0.00 |
| Group 1 (N = 42) | Group 2 (N = 38) | Group 3 (N = 35) | |
|---|---|---|---|
| CPU time for MCAE (ms) | 23.61 ± 0.87 | 20.93 ± 0.63 * | 21.62 ± 0.77 |
| CPU time for PEIoriginal (ms) | 14.17 ± 0.53 | 13.95 ± 0.78 | 13.65 ± 0.66 |
| CPU time for PEINEW (ms) | 3.80 ± 0.29 | 7.87 ± 0.33 ** | 8.11 ± 0.39 |
| MCAE | PEIoriginal | PEINEW | |
|---|---|---|---|
| Group 1 | 23.61 ± 0.87 | 14.17 ± 0.53 ** | 3.80 ± 0.29 †† |
| Group 2 | 20.93 ± 0.63 | 13.95 ± 0.78 * | 7.87 ± 0.33 † |
| Group 3 | 21.62 ± 0.77 | 13.65 ± 0.66 * | 8.11 ± 0.39 † |
| Group 2&Group 3 | 21.31 ± 0.69 | 13.81 ± 0.75 * | 7.95 ± 0.38 † |
| Group 1&Group 2&Group 3 | 22.03 ± 0.81 | 14.00 ± 0.65 ** | 5.88 ± 0.34 †† |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.-X.; Lu, C.-H.; Ta, N.; Jiang, W.-W.; Tang, X.-J.; Wu, H.-T. Application of a Speedy Modified Entropy Method in Assessing the Complexity of Baroreflex Sensitivity for Age-Controlled Healthy and Diabetic Subjects. Entropy 2019, 21, 894. https://doi.org/10.3390/e21090894
Xiao M-X, Lu C-H, Ta N, Jiang W-W, Tang X-J, Wu H-T. Application of a Speedy Modified Entropy Method in Assessing the Complexity of Baroreflex Sensitivity for Age-Controlled Healthy and Diabetic Subjects. Entropy. 2019; 21(9):894. https://doi.org/10.3390/e21090894
Chicago/Turabian StyleXiao, Ming-Xia, Chang-Hua Lu, Na Ta, Wei-Wei Jiang, Xiao-Jing Tang, and Hsien-Tsai Wu. 2019. "Application of a Speedy Modified Entropy Method in Assessing the Complexity of Baroreflex Sensitivity for Age-Controlled Healthy and Diabetic Subjects" Entropy 21, no. 9: 894. https://doi.org/10.3390/e21090894





