Increased Sample Entropy in EEGs During the Functional Rehabilitation of an Injured Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Photochemistry-Induced Cerebral Ischemia Model and Electrode Implantation
2.3. Motor Function Assessing
2.4. EEG Recording
2.5. EEG Signal Processing
2.5.1. Linear Analysis: Band Energy
2.5.2. Nonlinear Dynamic Analysis: ApEn, SampEn, and LZC
- Define m vectors A(1), A(2), A(3),…, A(N) according to the followingAm(i) = [a(i), a(i + 1), a(i + 2), . . . , a(i + m − 1)] for 1 ≤ i ≤ N − m + 1
- Calculate the maximum norm, which can be obtained from the distance between Am(i) and Am(y)
- Calculate the number of y (y = 1 ... N − m + 1, y ≠ i) according to the known A(i), provided that d [A(i), A(y)] ≤ r is expressed as Mm (i). Then, for i = N − m
- Calculate the natural logarithm of each and its average
- Get (e) and (e) through repeat step (1) to step (4), incrementing the dimension by one each time
- Finally, ApEn is calculated according the followingApEn(m,r, N) = Ψm(r) − Ψm+1(r)
- Calculate the natural logarithm of each , and its average
- Increase the dimension to m + 1, get (r) and (r) through Formulas (2)–(4) and (7)
- Finally, SampEn is computed by the following:
2.6. Statistical Analysis
3. Results
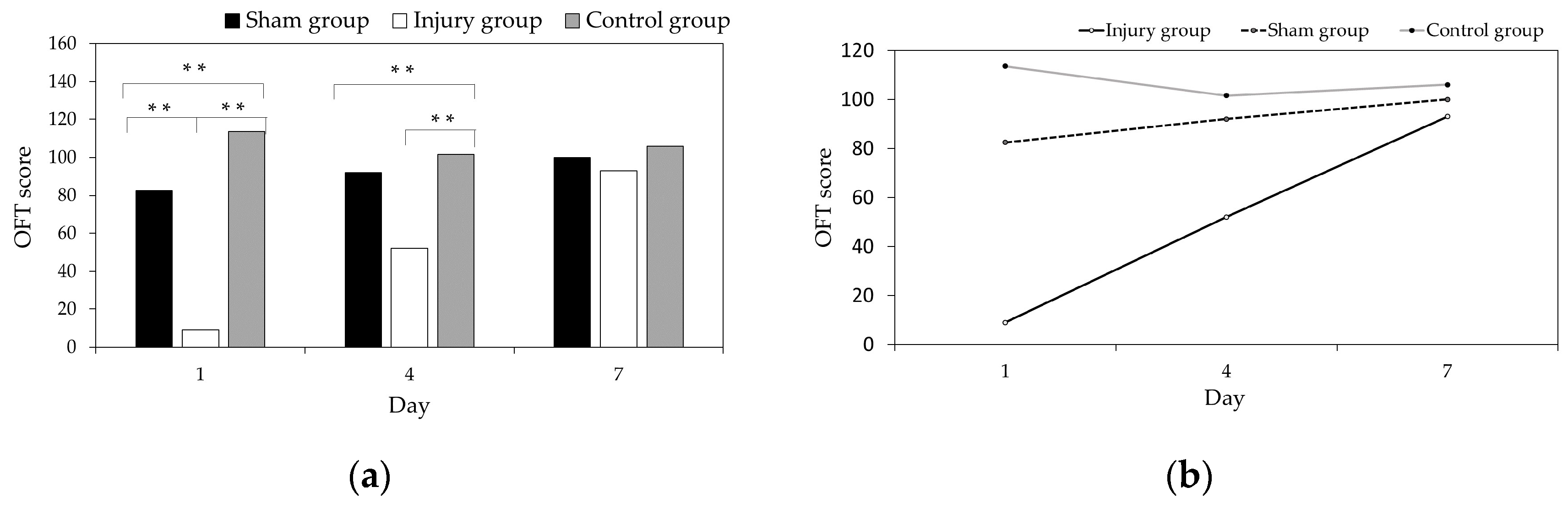
3.1. Comparative Analysis of the OFT Scores among the Injured Group, Sham Group, and Control Group
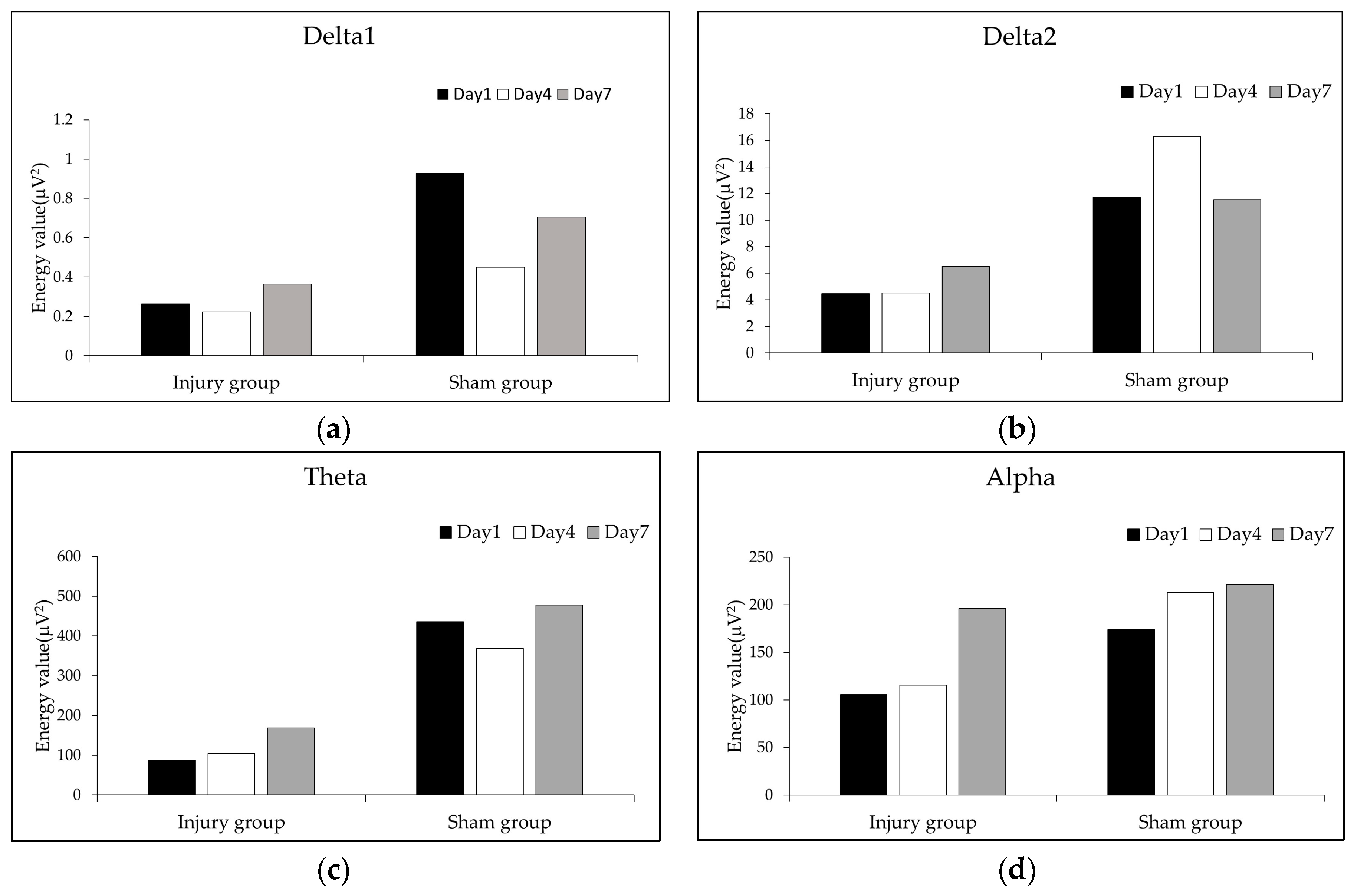
3.2. Comparative Analysis of EEG Signals in the Injured Area and the Symmetrical Non-Injured Area
3.3. Dynamic Change Analysis of EEG Signals in the Injured Area
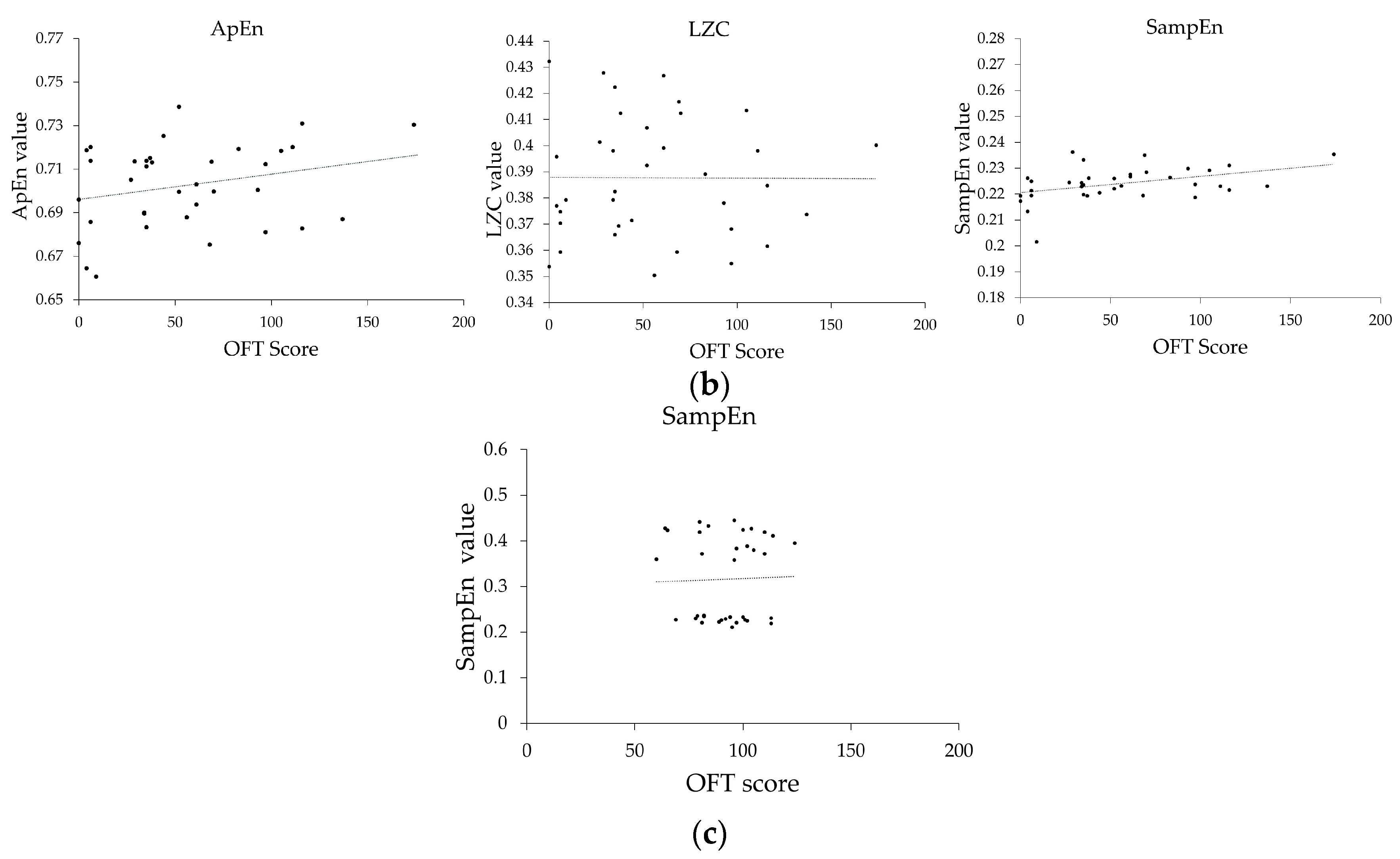
3.4. Relationship between EEG Features and Motor Function Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mukherjee, D.; Patil, C.G. Epidemiology and the Global Burden of Stroke. World Neurosurg. 2011, 76, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xu, H.; Wang, M.; Li, Z.; Su, X.; Li, X.; Li, Z.; Han, X. Effect of infusion speed of 7.5% hypertonic saline on brain edema in patients with craniocerebral injury: An experimental study. Gene 2018, 665, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Park, C.-H.; Kim, D.Y.; Shin, Y.-I.; Ko, M.-H.; Lee, A.; Jang, S.Y.; Kim, Y.-H. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC Neurol. 2016, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, K.; Ding, H.; Gao, H.; Zheng, X.; Ding, Z.; Xu, K.; Li, P. Longitudinal in vivo intrinsic optical imaging of cortical blood perfusion and tissue damage in focal photothrombosis stroke model. J. Cereb. Blood Flow. Metab. 2018, 39, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lee, J.; Fine, H.A. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J. Clin. Investig. 2004, 113, 1364–1374. [Google Scholar] [CrossRef] [Green Version]
- Rodger, J.; Sherrard, R.M. Optimising repetitive transcranial magnetic stimulation for neural circuit repair following traumatic brain injury. Neural Regen. Res. 2015, 10, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.; Friston, K. Analysis of fMRI Time-Series Revisited—Again. NeuroImage 1995, 2, 173–181. [Google Scholar] [CrossRef]
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 2000, 41, 1369–1379. [Google Scholar]
- Shayegh, F.; Sadri, S.; Amirfattahi, R.; Ansari-Asl, K. A model-based method for computation of correlation dimension, Lyapunov exponents and synchronization from depth-EEG signals. Comput. Methods Programs Biomed. 2014, 113, 323–337. [Google Scholar] [CrossRef]
- Cohen, B.A.; Bravo-Fernandez, E.J.; Sances, A. Automated electroencephalographic analysis as a prognostic indicator in stroke. Med. Biol. Eng. 1977, 15, 431–437. [Google Scholar] [CrossRef]
- Cohen, B.A.; Bravo-Fernandez, E.J.; Sances, A. Quantification of computer analyzed serial EEGs from stroke patients. Electroencephalogr. Clin. Neurophysiol. 1976, 41, 379–386. [Google Scholar] [CrossRef]
- Juhász, C.; Kamondi, A.; Szirmai, I. Spectral EEG analysis following hemispheric stroke: Evidences of transhemispheric diaschisis. Acta Neurol. Scand. 1997, 96, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Tucker, D.M.; Englander, R.; Lockfeld, A.; Lutsep, H.; Oken, B. Localizing acute stroke-related EEG changes: Assessing the effects of spatial undersampling. J. Clin. Neurophysiol. 2001, 18, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Esteller, R.; Echauz, J.; D’Alessandro, M.; Worrell, G.; Cranstoun, S.; Vachtsevanos, G.; Litt, B. Continuous energy variation during the seizure cycle: Towards an on-line accumulated energy. Clin. Neurophysiol. 2005, 116, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Gautama, T.; Mandic, D.P.; Van Hulle, M.M. Indications of nonlinear structures in brain electrical activity. Phys. Rev. E 2003, 67, 046204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, R.; Chen, Z.; Chang, X.; Chen, J.; Xie, L. Application of approximate entropy on dynamic characteristics of epileptic absence seizure. Neural Regen. Res. 2012, 7, 572–577. [Google Scholar]
- Abásolo, D.; Hornero, R.; Gómez, C.; García, M.; López, M. Analysis of EEG background activity in Alzheimer’s disease patients with Lempel–Ziv complexity and central tendency measure. Med. Eng. Phys. 2006, 28, 315–322. [Google Scholar] [CrossRef]
- Labate, D.; Foresta, F.L.; Mammone, N.; Morabito, F.C. Effects of Artifacts Rejection on EEG Complexity in Alzheimer’s Disease; Springer International Publishing: Reggio Calabria, Italy, 2015; Volume 47, pp. 46–52. ISBN 978-3-319-18163-9. [Google Scholar]
- Wu, H.J.; Zheng, C.X.; Zhang, J.W.; Zhang, H. In Nonlinear Entropy Analysis for Detecting Focal Cerebral Ischemia. In Proceedings of the IEEE EMBS Asian-Pacific Conference on Biomedical Engineering, Kyoto, Japan, 20–22 October 2003; pp. 182–183. [Google Scholar]
- Li, Y.; Tong, S.; Liu, D.; Gai, Y.; Wang, X.; Wang, X.; Qiu, Y.; Zhu, Y. Abnormal EEG complexity in patients with schizophrenia and depression. Clin. Neurophysiol. 2008, 119, 1232–1241. [Google Scholar] [CrossRef]
- Nurujjaman, M.; Narayanan, R.; Iyengar, A.S. Comparative study of nonlinear properties of EEG signals of normal persons and epileptic patients. Nonlinear Biomed. Phys. 2009, 3, 6. [Google Scholar] [CrossRef]
- Fan, J.; Fu, X.; Li, L.; Hao, X.; Li, S. Nonhuman primate models of focal cerebral ischemia. Neural Regen Res. 2017, 12, 321–328. [Google Scholar]
- Clément, Y.; Le Guisquet, A.-M.; Venault, P.; Chapouthier, G.; Belzung, C. Pharmacological Alterations of Anxious Behaviour in Mice Depending on Both Strain and the Behavioural Situation. PLoS ONE 2009, 4, e7745. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Lempel, A.; Ziv, J. On the Complexity of Finite Sequences. IEEE Trans. Inf. Theory 1976, 22, 75–81. [Google Scholar] [CrossRef]
- Kumar, Y.; Dewal, M.L.; Anand, R.S. Epileptic seizures detection in EEG using DWT-based ApEn and artificial neural network. Signal Image Video Process. 2014, 8, 1323–1334. [Google Scholar] [CrossRef]
- Ocak, H. Automatic detection of epileptic seizures in EEG using discrete wavelet transform and approximate entropy. Expert Syst. Appl. 2009, 36, 2027–2036. [Google Scholar] [CrossRef]
- Davey, M.P.; Victor, J.D.; Schiff, N.D. Power spectra and coherence in the EEG of a vegetative patient with severe asymmetric brain damage. Clin. Neurophysiol. 2000, 111, 1949–1954. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Liu, J.; Wang, J. Approximate entropy of EEG as a measure of cerebral ischemic injury. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 4537–4539. [Google Scholar]
- Chen, S.; Ma, X.; Chen, J.W.; Liu, Z.S. Determination of behavioral and cognitive dysfunction after moderate brain injury in rats. Chin. Tissue Eng. Res. 2004, 8, 48–49. (In Chinese) [Google Scholar]
- Li, Y.; Zhao, B.; Yang, W.; Li, J.; Xu, W.; Xu, Y.; Shen, C.; Li, L.; Yang, Y. Localization of brain injuries in vegetative and minimally conscious patients using symmetric electrode EEG analysis. Chin. Sci. Bull. 2013, 58, 2087–2093. [Google Scholar] [CrossRef]
- Yuan, D.G.; Zhang, F.C.; Wei, W.; Zheng, L. Research progress in neuroplasticity and recovery of motor function after stroke. Chin. J. Rehabil. Med. 2014, 29, 391–394. [Google Scholar]
- Michael, C.; Zhang, Z.G.; Quan, J. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke J. Cereb. Circ. 2007, 38, 827–831. [Google Scholar]
- Teng, H.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Zhang, L.; Morris, D.; R Gregg, S.; Wu, Z.; Lu, M.; Jiang, A.; et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2008, 28, 764–771. [Google Scholar] [CrossRef] [PubMed]





| Features of EEG Signal | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Delta1 | −0.184 | 0.283 |
| Delta2 | −0. 07 | 0.685 |
| Theta | 0.073 | 0.673 |
| Alpha | 0.217 | 0.204 |
| Beta1 | 0.209 | 0.221 |
| Beta2 | 0.217 | 0.203 |
| LZC | −0.006 | 0.972 |
| ApEn | 0.216 | 0.206 |
| SampEn | 0.393 | 0.018 * |
| ApEn (sham group) | −0.139 | 0.42 |
| SampEn (sham group) | −0.025 | 0.886 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Yang, W.; Liu, K.; Zhao, W.; Wu, L.; Lei, L.; Dong, T.; Hou, N.; Yang, F.; Qu, Y.; et al. Increased Sample Entropy in EEGs During the Functional Rehabilitation of an Injured Brain. Entropy 2019, 21, 698. https://doi.org/10.3390/e21070698
Cheng Q, Yang W, Liu K, Zhao W, Wu L, Lei L, Dong T, Hou N, Yang F, Qu Y, et al. Increased Sample Entropy in EEGs During the Functional Rehabilitation of an Injured Brain. Entropy. 2019; 21(7):698. https://doi.org/10.3390/e21070698
Chicago/Turabian StyleCheng, Qiqi, Wenwei Yang, Kezhou Liu, Weijie Zhao, Li Wu, Ling Lei, Tengfei Dong, Na Hou, Fan Yang, Yang Qu, and et al. 2019. "Increased Sample Entropy in EEGs During the Functional Rehabilitation of an Injured Brain" Entropy 21, no. 7: 698. https://doi.org/10.3390/e21070698





