A Novel Low-Activation VCrFeTaxWx (x = 0.1, 0.2, 0.3, 0.4, and 1) High-Entropy Alloys with Excellent Heat-Softening Resistance
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
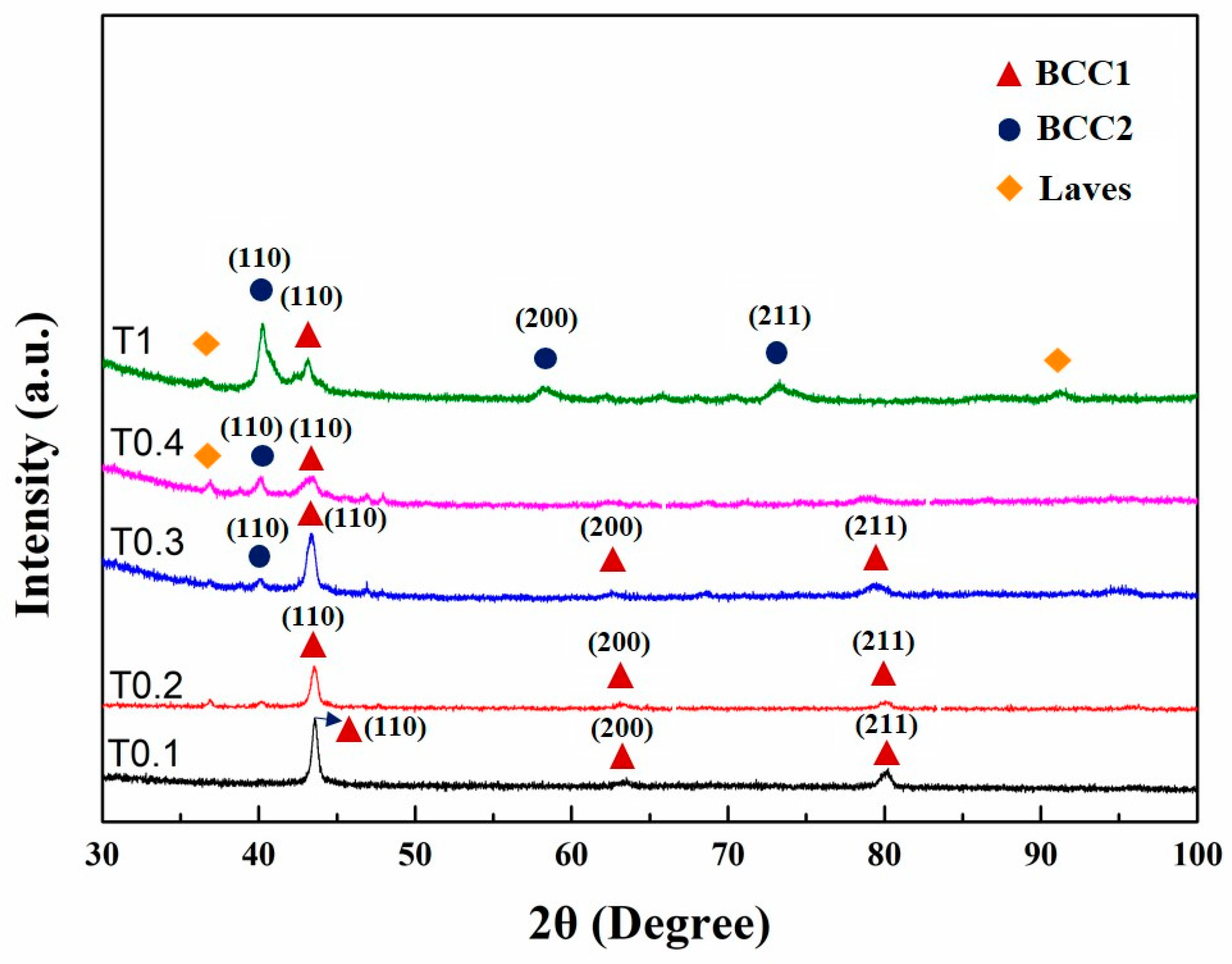
3.1. Structural Characterization
3.2. Microstructures and Chemical Compositions
3.3. Mechanical Properties
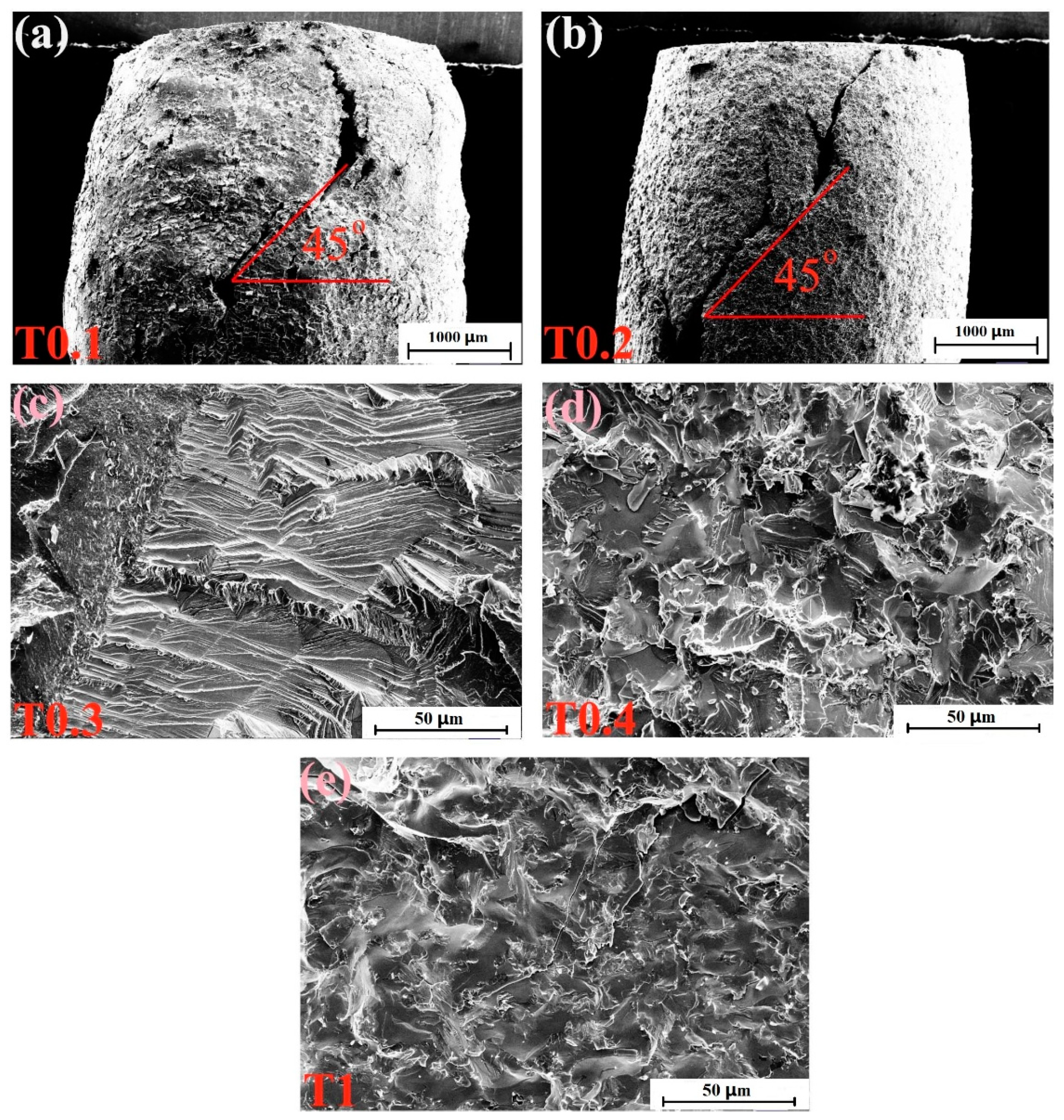
3.3.1. Mechanical Properties at Room Temperature
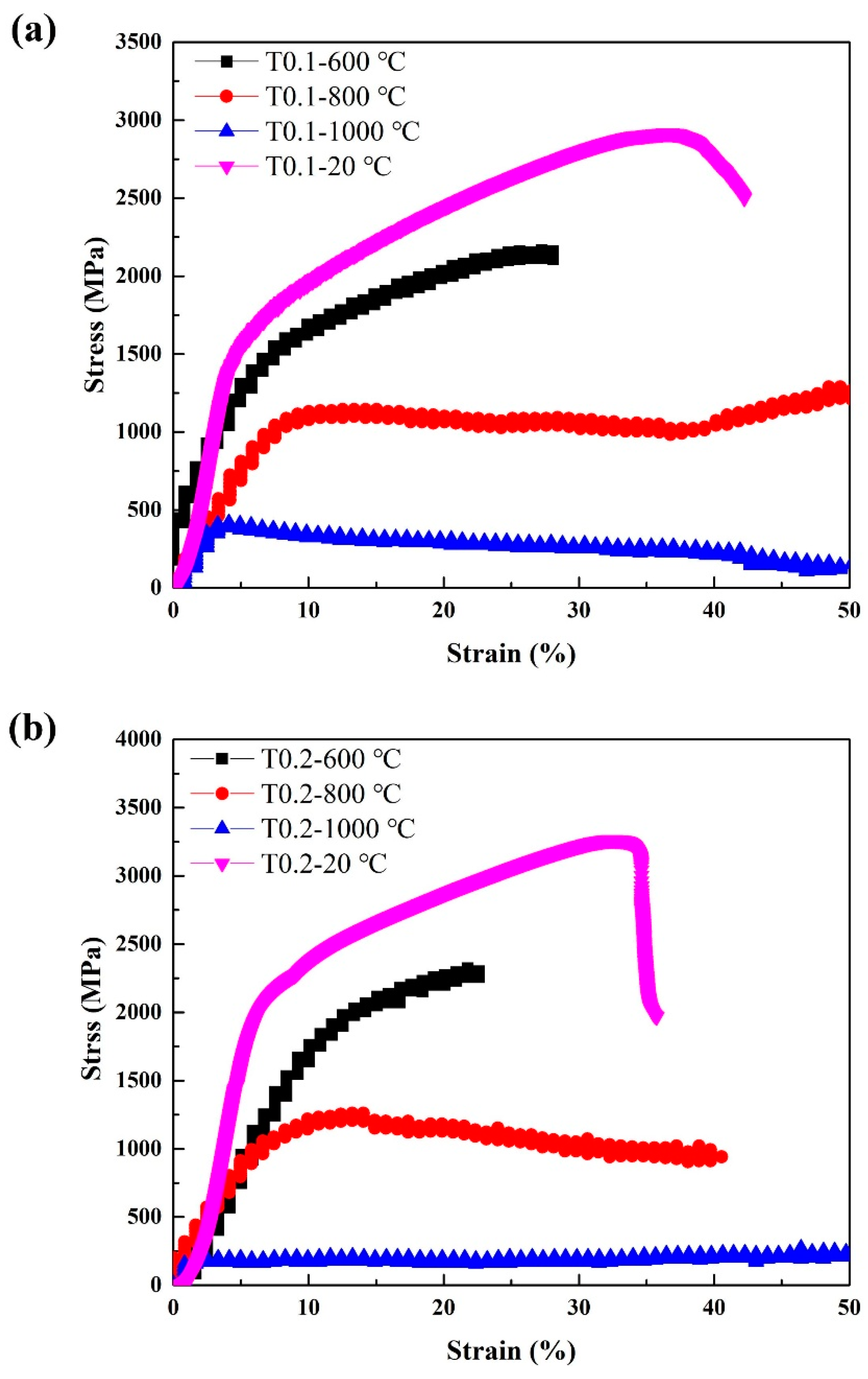
3.3.2. Mechanical Properties at High-Temperature
4. Discussion
4.1. Phase Selection
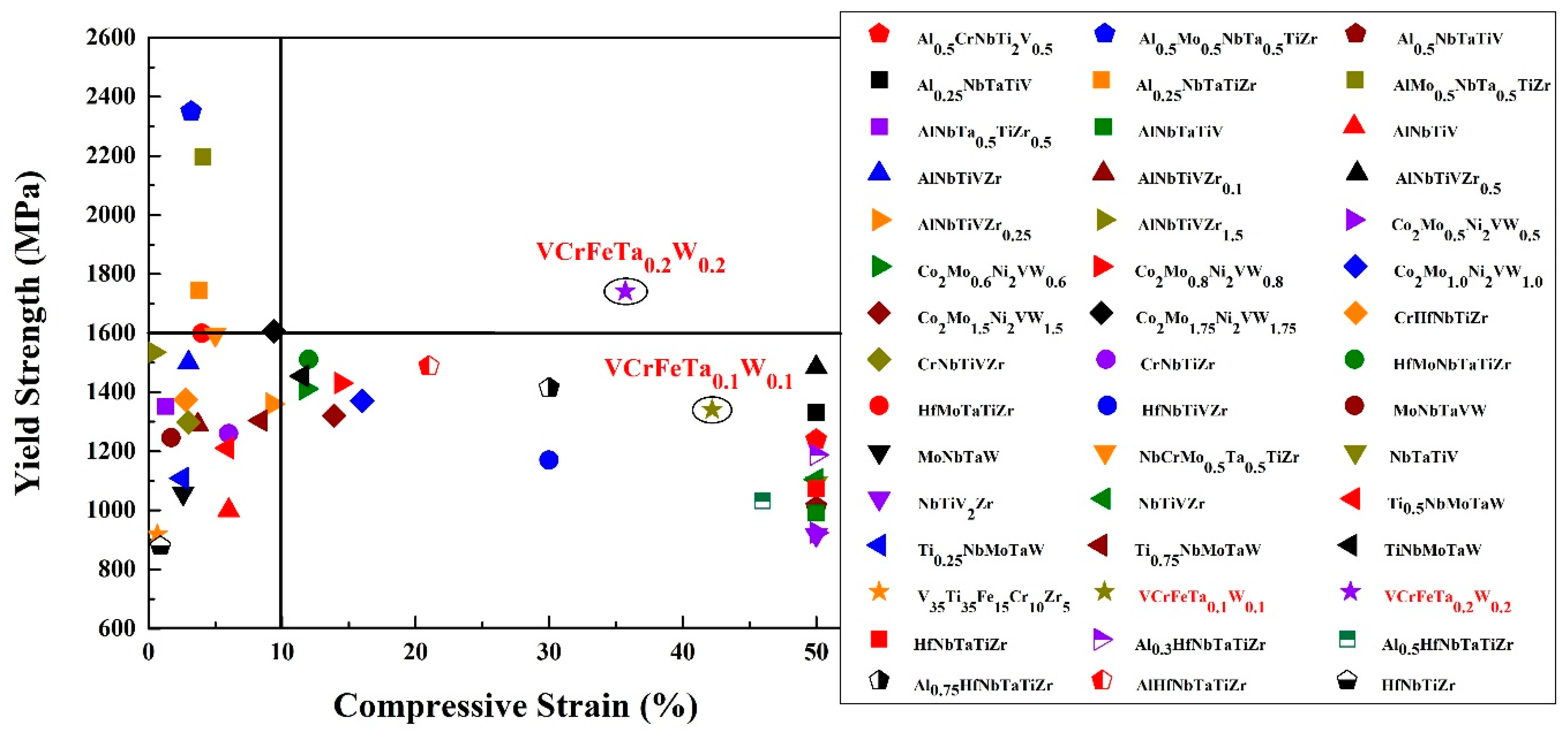
4.2. Ta and W Effects at Room Temperature
4.3. Heat-Softening Resistance
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Knaster, J.; Moeslang, A.; Muroga, T. Materials research for fusion. Nat. Phys. 2016, 12, 424. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Kanpara, S.; Khirwadkar, S.; Belsare, S.; Bhope, K.; Swamy, R.; Patil, Y.; Mokariya, P.; Patel, N.; Patel, T.; Galodiya, K. Fabrication of tungsten & tungsten alloy and its high heat load testing for fusion applications. Mater. Today Proc. 2016, 3, 3055–3063. [Google Scholar]
- Yan, Z.; Liu, S.; Xia, S.; Zhang, Y.; Wang, Y.; Yang, T. He behavior in Ni and Ni-based equiatomic solid solution alloy. J. Nuclear Mater. 2018, 505, 200–206. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Z.; Mo, K.; Wang, P.; Miao, Y.; Li, S.; Wang, M.; Liu, X.; Gong, M.; Almer, J. The microstructure and mechanical properties of Al-containing 9Cr ODS ferritic alloy. J. Alloys Compd. 2015, 648, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Lu, C.; Wang, L.M.; Qu, J.; Weber, W.J.; Zhang, Y.; Bei, H. Effects of compositional complexity on the ion-irradiation induced swelling and hardening in Ni-containing equiatomic alloys. Scr. Mater. 2016, 119, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Katoh, Y.; Tavassoli, A.A.F.; Henry, J.; Rieth, M.; Sakasegawa, H.; Tanigawa, H.; Huang, Q. Recent status and improvement of reduced-activation ferritic-martensitic steels for high-temperature service. J. Nuclear Mater. 2016, 479, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Snead, L.L.; Katoh, Y. Development of new generation reduced activation ferritic-martensitic steels for advanced fusion reactors. J. Nuclear Mater. 2016, 478, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Team, F. Development status of clam steel for fusion application. J. Nuclear Mater. 2014, 455, 649–654. [Google Scholar] [CrossRef]
- Abdou, M.; Morley, N.B.; Smolentsev, S.; Ying, A.; Malang, S.; Rowcliffe, A.; Ulrickson, M. Blanket/first wall challenges and required R&D on the pathway to DEMO. Fus. Eng. Des. 2015, 100, 2–43. [Google Scholar]
- Alimov, V.K.; Hatano, Y.; Yoshida, N.; Watanabe, H.; Oyaidzu, M.; Tokitani, M.; Hayashi, T. Surface modification and sputtering erosion of reduced activation ferritic martensitic steel F82H exposed to low-energy, high flux deuterium plasma. Nuclear Mater. Energy 2016, 7, 25–32. [Google Scholar] [CrossRef]
- Emmerich, T.; Qu, D.; Vaßen, R.; Aktaa, J. Development of W-coating with functionally graded W/EUROFER-layers for protection of First-Wall materials. Fus. Eng. Des. 2018, 128, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Xian, X.; Zhong, Z.; Zhang, B.; Song, K.; Chen, C.; Wang, S.; Cheng, J.; Wu, Y. A high-entropy V35TI35FE15Cr10Zr5 alloy with excellent high-temperature strength. Mater. Des. 2017, 121, 229–236. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation resistance in AlxCoCrFeNi high entropy alloys. JOM 2015, 67, 2340–2344. [Google Scholar] [CrossRef]
- Xia, S.Q.; Gao, M.C.; Yang, T.F.; Liaw, P.K.; Zhang, Y. Phase stability and microstructures of high entropy alloys ion irradiated to high doses. J. Nuclear Mater. 2016, 480, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lee, D.-H.; Seok, M.-Y.; Lee, J.-A.; Phaniraj, M.; Suh, J.-Y.; Ha, H.-Y.; Kim, J.-Y.; Ramamurty, U.; Jang, J.-I. Resistance of cocrfemnni high-entropy alloy to gaseous hydrogen embrittlement. Scr. Mater. 2017, 135, 54–58. [Google Scholar] [CrossRef]
- Hosemann, P.; Frazer, D.; Fratoni, M.; Bolind, A.; Ashby, M. Materials selection for nuclear applications: Challenges and opportunities. Scr. Mater. 2018, 143, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Progr. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin, Germany, 2016. [Google Scholar]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.; Fu, Z.; Zheng, B.; Chen, W.; Lin, Y.; Chen, F.; Zhang, L.; Ivanisenko, J.; Zhou, Y.; Hahn, H. Recent progress in high entropy alloy research. JOM 2017, 69, 2024–2031. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 1–37. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of tixnbmotaw refractory high-entropy alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.-P.; Brenner, D.W.; Vecchio, K.S.; Curtarolo, S. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 2018, 9, 4980. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Konarski, P.; Misnik, M.; Ivashchenko, V.I.; Kempiński, M.; Mediukh, N.R.; Pogrebnjak, A.D.; Beresnev, V.M.; et al. A new type of (TiZrNbTaHf)N/MoN nanocomposite coating: Microstructure and properties depending on energy of incident ions. Compos. B Eng. 2018, 146, 132–144. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef] [Green Version]
- Schuh, B.; Völker, B.; Maier-Kiener, V.; Todt, J.; Li, J.; Hohenwarter, A. Phase decomposition of a single-phase AlTiVNb high-entropy alloy after severe plastic deformation and annealing. Adv. Eng. Mater. 2017, 19, 1600674. [Google Scholar] [CrossRef]
- Kumar, N.A.P.K.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of fenimncr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef]
- Zou, Y.; Wheeler, J.M.; Ma, H.; Okle, P.; Spolenak, R. Nanocrystalline high-entropy alloys: A new paradigm in high-temperature strength and stability. Nano Lett. 2017, 17, 1569–1574. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, J.; Xia, Z.; Fu, W.; Wu, K.; Liu, G.; Sun, J. Stable nanocrystalline nbmotaw high entropy alloy thin films with excellent mechanical and electrical properties. Mater. Lett. 2018, 210, 84–87. [Google Scholar] [CrossRef]
- Sathiyamoorthi, P.; Basu, J.; Kashyap, S.; Pradeep, K.; Kottada, R.S. Thermal stability and grain boundary strengthening in ultrafine-grained cocrfeni high entropy alloy composite. Mater. Des. 2017, 134, 426–433. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Diao, H.Y.; Gao, M.C.; Tang, Z.; Poplawsky, J.D.; Liaw, P.K. Understanding phase stability of Al-Co-Cr-Fe-Ni high entropy alloys. Mater. Des. 2016, 109, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.C.; Diao, H.Y.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.D.; Zhou, Y. Secondary phases in AlxCoCrFeNi high-entropy alloys: An in-situ tem heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, L.; Luo, L.S.; Li, X.Z.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of refractory MoNbHfZrTi high-entropy alloy. Mater. Des. 2015, 81, 87–94. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Xing, Q.; Ma, J.; Wang, C.; Zhang, Y. High-throughput screening solar-thermal conversion films in a pseudobinary (Cr, Fe, V)–(Ta, W) system. ACS Comb. Sci. 2018, 20, 602–610. [Google Scholar] [CrossRef]
- Hasegawa, A.; Fukuda, M.; Yabuuchi, K.; Nogami, S. Neutron irradiation effects on the microstructural development of tungsten and tungsten alloys. J. Nuclear Mater. 2016, 471, 175–183. [Google Scholar] [CrossRef]
- Waseem, O.A.; Lee, J.; Lee, H.M.; Ryu, H.J. The effect of ti on the sintering and mechanical properties of refractory high-entropy alloy TixWTaVCr fabricated via spark plasma sintering for fusion plasma-facing materials. Mater. Chem. Phys. 2018, 210, 87–94. [Google Scholar] [CrossRef]
- Rieth, M.; Dudarev, S.; De Vicente, S.G.; Aktaa, J.; Ahlgren, T.; Antusch, S.; Armstrong, D.; Balden, M.; Baluc, N.; Barthe, M.-F. Recent progress in research on tungsten materials for nuclear fusion applications in europe. J. Nuclear Mater. 2013, 432, 482–500. [Google Scholar] [CrossRef]
- Xiao, D.H.; Zhou, P.F.; Wu, W.Q.; Diao, H.Y.; Gao, M.C.; Song, M.; Liaw, P.K. Microstructure, mechanical and corrosion behaviors of AlCoCuFeNi-(Cr,Ti) high entropy alloys. Mater. Des. 2017, 116, 438–447. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; Shanker Rajamure, R.; Dahotre, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.Y.; Lu, Y.P.; Gao, X.X.; Wang, T.M.; Li, T.J. Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.M.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Diao, H.Y.; Feng, R.; Dahmen, K.A.; Liaw, P.K. Fundamental deformation behavior in high-entropy alloys: An overview. Curr. Opin. Solid State Mater. Sci. 2017, 21, 252–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wu, Y.; Lou, H.B.; Zeng, Z.D.; Prakapenka, V.B.; Greenberg, E.; Ren, Y.; Yan, J.Y.; Okasinski, J.S.; Liu, X.J. Polymorphism in a high-entropy alloy. Nat. Commun. 2017, 8, 15687. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Steurer, W. Structural-disorder and its effect on mechanical properties in single-phase TaNbHfZr high-entropy alloy. Acta Mater. 2016, 106, 87–97. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-transformation ductilization of brittle high-entropy alloys via metastability engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.F. Microstructure and properties of a refractory NbCrMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Juan, C.C.; Tsai, M.H.; Tsai, C.W.; Lin, C.M.; Wang, W.R.; Yang, C.C.; Chen, S.K.; Lin, S.J.; Yeh, J.W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Senkov, O.; Jensen, J.; Pilchak, A.; Miracle, D.; Fraser, H.J. Compositional variation effects on the microstructure and properties of a refractory high-entropy superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Miracle, D.B.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and properties of aluminum-containing refractory high-entropy alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Lin, C.M.; Juan, C.C.; Chang, C.H.; Tsai, C.W.; Yeh, J.W. Effect of Al addition on mechanical properties and microstructure of refractory AlxHfNbTaTIZr alloys. J. Alloys Compd. 2015, 624, 100–107. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Wang, T.; Si, J.J.; Zhu, J.; Wang, Y.D.; Hui, X.D. A refractory Hf25Nb25Ti25Zr25 high-entropy alloy with excellent structural stability and tensile properties. Mater. Lett. 2014, 130, 277–280. [Google Scholar] [CrossRef]
- Senkov, O.N.; Jensen, J.K.; Pilchak, A.L.; Miracle, D.B.; Fraser, H.L. Compositional variation effects on the microstructure and properties of a refractory high-entropy superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2017, 139, 498–511. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Panina, E.S.; Tikhonovsky, M.A.; Zherebtsov, S.V. Precipitation-strengthened refractory Al0.5CrNbTi2V0.5 high entropy alloy. Mater. Lett. 2017, 188, 162–164. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and mechanical properties of B2 ordered refractory AlNbTiVZrx (x = 0–1.5) high-entropy alloys. Mater. Sci. Eng. A 2017, 704, 82–90. [Google Scholar] [CrossRef]
- Sheikh, S.; Shafeie, S.; Hu, Q.; Ahlström, J.; Persson, C.; Veselý, J.; Zýka, J.; Klement, U.; Guo, S. Alloy design for intrinsically ductile refractory high-entropy alloys. J. Appl. Phys. 2016, 120, 3445. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Liaw, P.K. Microstructure and compressive properties of NbTiVTaAlx high entropy alloys. Procedia Eng. 2012, 36, 292–298. [Google Scholar] [CrossRef]
- Senkov, O.N.; Zhang, F.; Miller, J.D. Phase composition of a CrMo0.5NbTa0.5TiZr high entropy alloy: Comparison of experimental and simulated data. Entropy 2013, 15, 3796–3809. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Huang, T.; Lu, Y.; Wang, T.; Li, T. Microstructures and mechanical properties of Co2MoxNi2VWx eutectic high entropy alloys. Mater. Des. 2016, 109, 539–546. [Google Scholar] [CrossRef]





| Low activation element | Fe | Ti | Cr | V | Ta | Zr | W | Mn | Si | Al | B | C | N | O |
| High activation element | Nb | Ni | Co | Cu | Mo |
| Alloy | Identification | Fe | Cr | V | Ta | W |
|---|---|---|---|---|---|---|
| VCrFeTa0.1W0.1 | T0.1 | 31.2 | 31.2 | 31.2 | 3.2 | 3.2 |
| VCrFeTa0.2W0.2 | T0.2 | 29.4 | 29.3 | 29.3 | 6 | 6 |
| VCrFeTa0.3W0.3 | T0.3 | 27.8 | 27.7 | 27.7 | 8.4 | 8.4 |
| VCrFeTa0.4W0.4 | T0.4 | 26 | 26 | 26 | 11 | 11 |
| VCrFeTaW | T1 | 20 | 20 | 20 | 20 | 20 |
| Alloy | Phase Composition | Lattice Constant (nm) |
|---|---|---|
| VCrFeTa0.1W0.1 | BCC1 | 0.2935 |
| VCrFeTa0.2W0.2 | BCC1 | 0.2937 |
| VCrFeTa0.3W0.3 | BCC1 | 0.2947 |
| BCC2 | 0.3174 | |
| VCrFeTa0.4W0.4 | BCC1 | 0.2963 |
| BCC2 | 0.3178 | |
| Laves | - | |
| VCrFeTaW | BCC1 | 0.2962 |
| BCC2 | 0.3166 | |
| Laves | - |
| Alloy | Region | V | Cr | Fe | Ta | W | |
|---|---|---|---|---|---|---|---|
| VCrFeTa0.1W0.1 | Overall | 33.29 ± 0.29 | 30.94 ± 0.17 | 30.75 ± 0.22 | 2.54 ± 0.27 | 2.47 ± 0.02 | |
| IR | White | 19.66 ± 0.30 | 22.26 ± 0.35 | 37.57 ± 0.52 | 20.51 ± 0.79 | / | |
| DR | Gray | 35.98 ± 0.30 | 33.32 ± 0.35 | 24.80 ± 0.52 | 2.20 ± 0.24 | 3.70 ± 0.40 | |
| VCrFeTa0.2W0.2 | Overall | 31.15 ± 0.21 | 29.97 ± 0.31 | 28.58 ± 0.40 | 5.01 ± 0.59 | 5.29 ± 0.08 | |
| IR | White | 15.98 ± 0.18 | 23.30 ± 0.21 | 38.75 ± 0.32 | 21.96 ± 0.49 | / | |
| DR | Gray | 35.57 ± 0.21 | 33.72 ± 0.19 | 21.95 ± 0.21 | 2.76 ± 0.31 | 6 ± 0.07 | |
| DR | Black | 32.41 ± 0.18 | 26.72 ± 0.16 | 39.49 ± 0.24 | 1.39 ± 0.11 | / | |
| VCrFeTa0.3W0.3 | Overall | 30.27 ± 0.20 | 28.54 ± 0.19 | 28.52 ± 0.52 | 6.21 ± 0.37 | 6.36 ± 0.04 | |
| IR | White | 10.33 ± 0.25 | 22.54 ± 0.62 | 42.89 ± 0.61 | 24.24 ± 0.14 | / | |
| DR | Gray | 24.75 ± 0.28 | 31.51 ± 0.59 | 31.36 ± 0.60 | / | 12.38 ± 0.10 | |
| DR | Black | 44.96 ± 0.36 | 23.82 ± 0.54 | 25.74 ± 0.65 | 5.48 ± 0.07 | / | |
| VCrFeTa0.4W0.4 | Overall | 27.54 ± 0.20 | 27.57 ± 0.21 | 27.19 ± 0.26 | 9.09 ± 0.43 | 8.62 ± 0.07 | |
| IR | White | 15.19 ± 0.28 | 23.59 ± 0.66 | 36.01 ± 0.63 | 25.20 ± 0.15 | / | |
| DR | Gray | 28.49 ± 0.54 | 26.94 ± 0.66 | 25.51 ± 0.62 | / | 19.06 ± 0.13 | |
| DR | Black | 28.88 ± 0.28 | 28.43 ± 0.52 | 36.17 ± 0.60 | / | 6.52 ± 0.07 | |
| VCrFeTaW | Overall | 22.12 ± 0.34 | 18.99 ± 0.71 | 20.99 ± 0.65 | 19.00 ± 0.82 | 18.90 ± 0.76 | |
| DR | White | 13.08 ± 0.50 | 7.76 ± 0.59 | / | / | 79.16 ± 0.39 | |
| IR | Gray | 14.64 ± 0.30 | 22.23 ± 0.41 | 30.96 ± 0.63 | 32.22 ± 0.18 | / | |
| IR | Black | 39.21 ± 0.60 | 25.32 ± 0.69 | 35.48 ± 0.76 | / | / | |
| Fe | Cr | V | Ta | W | |
|---|---|---|---|---|---|
| Fe | - | −1 | −7 | −15 | 0 |
| Cr | - | - | −2 | −7 | 1 |
| V | - | - | - | −1 | −1 |
| Ta | - | - | - | - | −7 |
| W | - | - | - | - | - |
| Alloy | Vickers Hardness (HV0.2) | |||
|---|---|---|---|---|
| VCrFeTa0.1W0.1 | 564 | 1341 | 2917 | 42.2 (Not broken) |
| VCrFeTa0.2W0.2 | 673 | 1742 | 3265 | 35.7 (Not broken) |
| VCrFeTa0.3W0.3 | 726 | / | 701 | / |
| VCrFeTa0.4W0.4 | 886 | 1580 | 1767 | / |
| VCrFeTaW | 1135 | / | 1501 | / |
| Alloy | Temperature (°C) | σ0.2(MPa) | MPa | εp (%) | Vickers Hardness (HV0.2) |
|---|---|---|---|---|---|
| VCrFeTa0.1W0.1 | 600 | 1234 | 2158 | 28.1 | 605 |
| 800 | 1019 | 1289 | > 50 | 621 | |
| 1000 | 371 | 421 | > 50 | 538 | |
| VCrFeTa0.2W0.2 | 600 | 1657 | 2316 | 22.6 | 721 |
| 800 | 1033 | 1260 | 40.6 | 762 | |
| 1000 | 182 | 253 | > 50 | 665 |
| Alloy | δ | ΔHmix (KJ/mol) | ΔSmix (J/mol·K) | VEC | ρtheor (g/cm3) | Tm (K) | |
|---|---|---|---|---|---|---|---|
| VCrFeTa0.1W0.1 | 3.59 | –4.83 | 10.87 | 4.83 | 6.28 | 7.85 | 2147.7 |
| VCrFeTa0.2W0.2 | 4.17 | –5.15 | 11.75 | 5.08 | 6.23 | 8.58 | 2227.3 |
| VCrFeTa0.3W0.3 | 4.55 | –5.41 | 12.32 | 5.23 | 6.19 | 9.19 | 2297.0 |
| VCrFeTa0.4W0.4 | 4.82 | –5.63 | 12.70 | 5.32 | 6.16 | 9.72 | 2360.1 |
| VCrFeTaW | 5.41 | –6.4 | 13.38 | 5.47 | 6 | 11.81 | 2631.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liaw, P.K.; Zhang, Y. A Novel Low-Activation VCrFeTaxWx (x = 0.1, 0.2, 0.3, 0.4, and 1) High-Entropy Alloys with Excellent Heat-Softening Resistance. Entropy 2018, 20, 951. https://doi.org/10.3390/e20120951
Zhang W, Liaw PK, Zhang Y. A Novel Low-Activation VCrFeTaxWx (x = 0.1, 0.2, 0.3, 0.4, and 1) High-Entropy Alloys with Excellent Heat-Softening Resistance. Entropy. 2018; 20(12):951. https://doi.org/10.3390/e20120951
Chicago/Turabian StyleZhang, Weiran, Peter K. Liaw, and Yong Zhang. 2018. "A Novel Low-Activation VCrFeTaxWx (x = 0.1, 0.2, 0.3, 0.4, and 1) High-Entropy Alloys with Excellent Heat-Softening Resistance" Entropy 20, no. 12: 951. https://doi.org/10.3390/e20120951







