Elemental Phase Partitioning in the γ-γ″ Ni2CoFeCrNb0.15 High Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
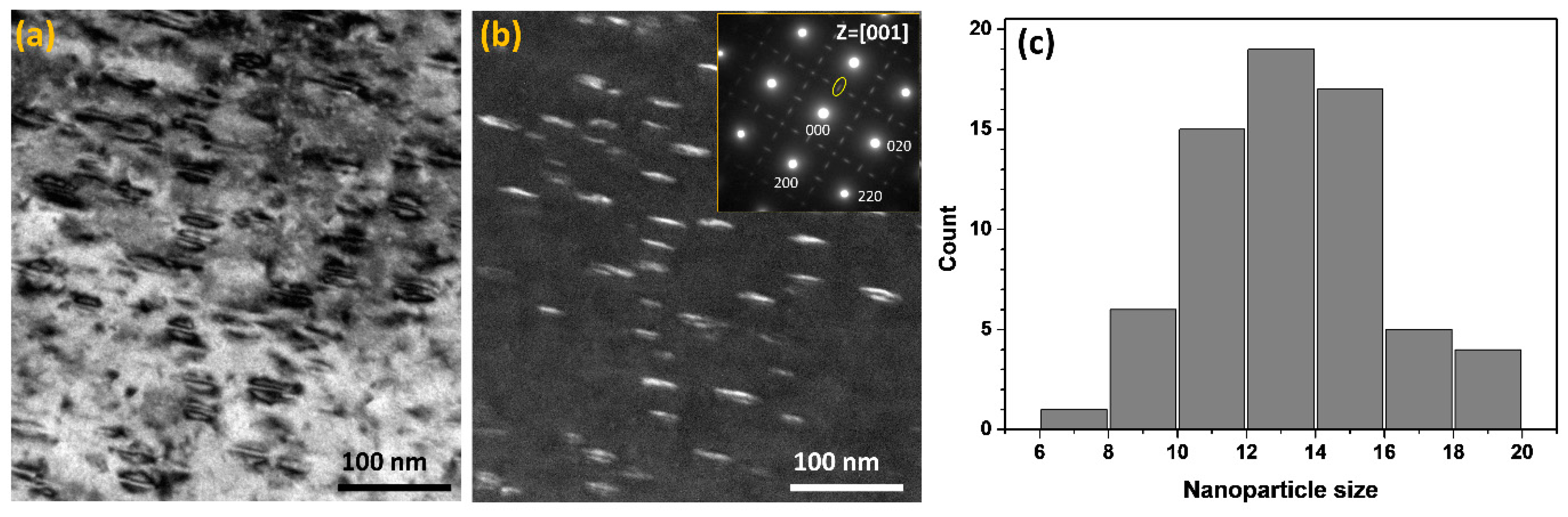
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sheng, H.; Wang, Z.; Gludovatz, B.; Zhang, Z.; George, E.P.; Yu, Q.; Mao, S.X.; Ritchie, R.O. Dislocation mechanisms and 3D twin architectures generate exceptional strength-ductility-toughness combination in CrCoNi medium-entropy alloy. Nat. Commun. 2017, 8, 14390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, C.L.; Park, S.; Rittman, D.R.; Zinkle, S.J.; Bei, H.; Lang, M.; Ewing, R.C.; Mao, W.L. High pressure synthesis of a hexagonal close-packed phase of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2017, 8, 15634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-Transformation Ductilization of Brittle High-Entropy Alloys via Metastability Engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Jung, S.; Choi, W.M.; Sohn, S.S.; Kim, H.S.; Lee, B.J.; Kim, N.J.; Lee, S. Cryogenic strength improvement by utilizing room-temperature deformation twinning in a partially recrystallized VCrMnFeCoNi high-entropy alloy. Nat. Commun. 2017, 8, 15719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Wang, Z.; Wu, Q.; Li, J.; Wang, J.; Liu, C.T. Phase separation of metastable CoCrFeNi high entropy alloy at intermediate temperatures. Scr. Mater. 2017, 126, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.C.; Diao, H.Y.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.D.; Zhou, Y.; et al. Secondary phases in AlxCoCrFeNi high-entropy alloys: An in-situ TEM heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Wang, L.; Wen, Y.; Cheng, B.; Wu, Q.; Cao, T.; Xiao, Q.; Xue, Y.; Sha, G.; Wang, Y.; et al. High-content ductile coherent nanoprecipitates achieve ultrastrong high-entropy alloys. Nat. Commun. 2018, 9, 4063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Thurston, K.V.; Bei, H.; Wu, Z.; George, E.P.; Ritchie, R.O. Exceptional damage-tolerance of a medium-entropy alloy CrCoNi at cryogenic temperatures. Nat. Commun. 2016, 7, 10602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Liu, W.H.; Zhu, J.H.; Kai, J.J.; Liu, C.T. Ductilizing brittle high-entropy alloys via tailoring valence electron concentrations of precipitates by controlled elemental partitioning. Mater. Res. Lett. 2018, 6, 600–606. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yang, T.; Tong, Y.; Wang, J.; Luan, J.H.; Jiao, Z.B.; Chen, D.; Yang, Y.; Hu, A.; Liu, C.T.; et al. Heterogeneous precipitation behavior and stacking-fault-mediated deformation in a CoCrNi-based medium-entropy alloy. Acta Mater. 2017, 138, 72–82. [Google Scholar] [CrossRef]
- Thomas, A.; El-Wahabi, M.; Cabrera, J.M.; Prado, J.M. High temperature deformation of Inconel 718. J. Mater. Process. Technol. 2006, 177, 469–472. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.M.; Wanderka, N.; Glatzel, U. High-Temperature Tensile Strength of Al10Co25Cr8Fe15Ni36Ti6 Compositionally Complex Alloy (High-Entropy Alloy). JOM 2015, 67, 2271–2277. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Yu, C.Y.; Liu, Y.; Liu, C.T. Precipitate transformation from NiAl-type to Ni 2 AlMn-type and its influence on the mechanical properties of high-strength steels. Acta Mater. 2016, 110, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Q.; Francis, E.; Robson, J.; Preuss, M.; Haigh, S.J. Compositional variations for small-scale gamma prime (γ′) precipitates formed at different cooling rates in an advanced Ni-based superalloy. Acta Mater. 2015, 85, 199–206. [Google Scholar] [CrossRef]
- Wang, W.Z.; Jin, T.; Jia, J.H.; Liu, J.L.; Hu, Z.Q. Effects of cobalt on creep rupture properties and dislocation structures in nickel base superalloys. Mater. Sci. Eng. A 2015, 624, 220–228. [Google Scholar] [CrossRef]
- Han, B.; Shimizu, Y.; Seguini, G.; Arduca, E.; Castro, C.; Ben Assayag, G.; Inoue, K.; Nagai, Y.; Schamm-Chardon, S.; Perego, M. Evolution of shape, size, and areal density of a single plane of Si nanocrystals embedded in SiO2 matrix studied by atom probe tomography. RSC Adv. 2016, 6, 3617–3622. [Google Scholar] [CrossRef]
- Han, B.; Wei, J.; Tong, Y.; Chen, D.; Zhao, Y.; Wang, J.; He, F.; Yang, T.; Zhao, C.; Shimizu, Y.; et al. Composition evolution of gamma prime nanoparticles in the Ti-doped CoFeCrNi high entropy alloy. Scr. Mater. 2018, 148, 42–46. [Google Scholar] [CrossRef]
- Miller, M.; Russell, K.; Thompson, G. Strategies for fabricating atom probe specimens with a dual beam FIB. Ultramicroscopy 2005, 102, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Vurpillot, F.; Gault, B.; Geiser, B.P.; Larson, D. Reconstructing atom probe data: A review. Ultramicroscopy 2013, 132, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lawitzki, R.; Hassan, S.; Karge, L.; Wagner, J.; Wang, D.; von Kobylinski, J.; Krempaszky, C.; Hofmann, M.; Gilles, R.; Schmitz, G. Differentiation of γ′- and γ″-precipitates in Inconel 718 by a complementary study with small-angle neutron scattering and analytical microscopy. Acta Mater. 2019, 163, 28–39. [Google Scholar] [CrossRef]
- Theska, F.; Stanojevic, A.; Oberwinkler, B.; Ringer, S.P.; Primig, S. On conventional versus direct ageing of Alloy 718. Acta Mater. 2018, 156, 116–124. [Google Scholar] [CrossRef]
- Kusabiraki, K.; Komatsu, H.; Ikeuchi, S. Lattice constants and compositions of the metastable Ni3Nb phase precipitated in a Ni-15Cr-8Fe-6Nb alloy. Metall. Mater. Trans. A 1998, 29, 1169–1174. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Connétable, D.; Galliano, F.; Odemer, G.; Blanc, C.; Andrieu, É. DFT study of the solubility of hydrogen and carbon in Ni3Nb-D0a and Ni3Nb-D022 systems. J. Alloys Compd. 2014, 610, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Booth-Morrison, C.; Mao, Z.; Noebe, R.D.; Seidman, D.N. Chromium and tantalum site substitution patterns in Ni3Al(L12) γ′-precipitates. Appl. Phys. Lett. 2008, 93, 033103. [Google Scholar] [CrossRef]
- Xu, J.-H.; Lin, W.; Freeman, A.J. Electronic structure and phase stability of A3Ti (A = Fe, Co, Ni, and Cu). Phys. Rev. B 1993, 48, 4276–4286. [Google Scholar] [CrossRef]
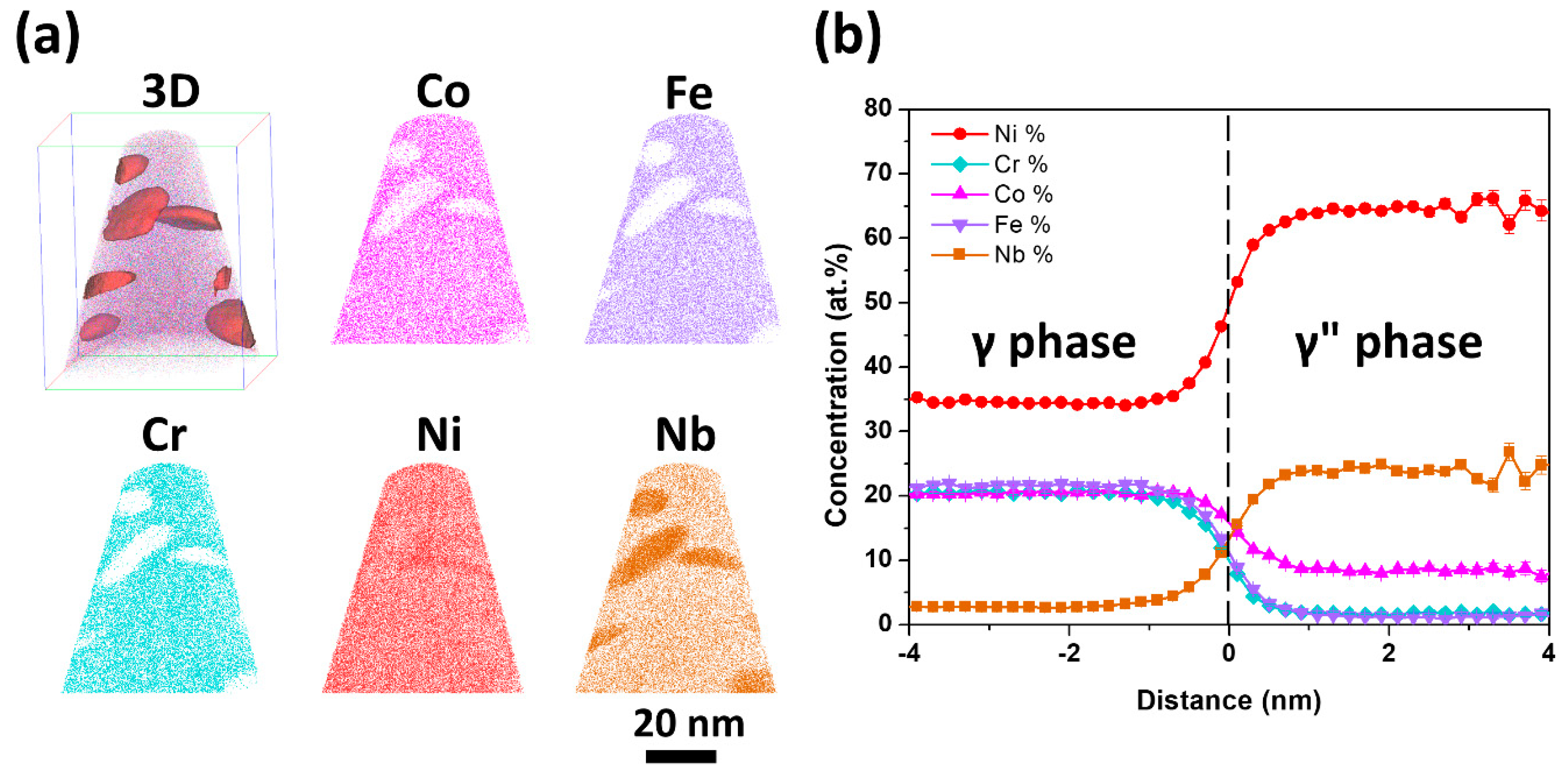
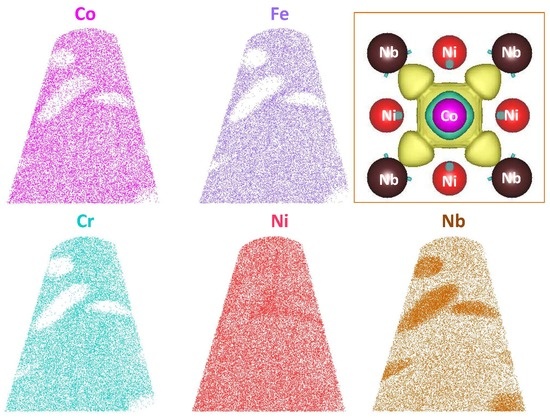
| Aging Time | Co | Fe | Cr | Ni | Nb |
|---|---|---|---|---|---|
| 40 h | 8.2 ± 0.3 | 1.3 ± 0.1 | 1.6 ± 0.1 | 64.3 ± 0.4 | 24.6 ± 0.2 |
| 100 h | 8.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 65.2 ± 0.3 | 24.0 ± 0.2 |
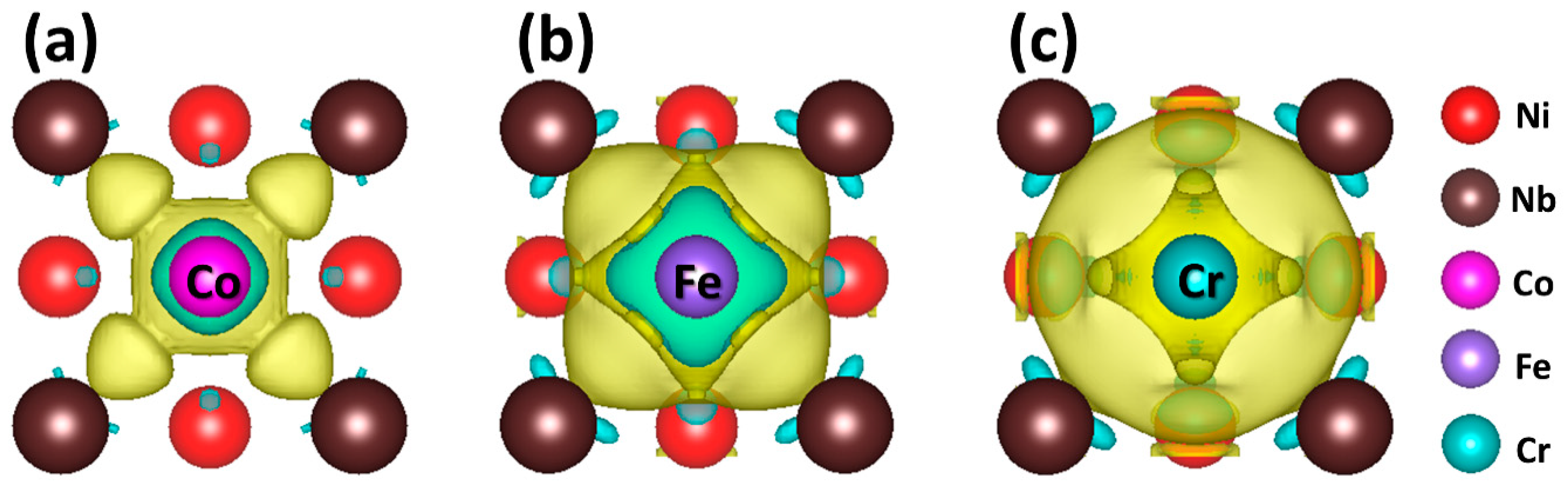
| μ | |||||
|---|---|---|---|---|---|
| Co | −7.01 | −224.16 | −217.74 | 0.08 | 1.71 |
| Fe | −8.23 | −225.00 | −218.79 | 0.19 | 1.61 |
| Cr | −9.50 | −225.55 | 220.65 | 1.38 | 1.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Wei, J.; He, F.; Chen, D.; Wang, Z.J.; Hu, A.; Zhou, W.; Kai, J.J. Elemental Phase Partitioning in the γ-γ″ Ni2CoFeCrNb0.15 High Entropy Alloy. Entropy 2018, 20, 910. https://doi.org/10.3390/e20120910
Han B, Wei J, He F, Chen D, Wang ZJ, Hu A, Zhou W, Kai JJ. Elemental Phase Partitioning in the γ-γ″ Ni2CoFeCrNb0.15 High Entropy Alloy. Entropy. 2018; 20(12):910. https://doi.org/10.3390/e20120910
Chicago/Turabian StyleHan, Bin, Jie Wei, Feng He, Da Chen, Zhi Jun Wang, Alice Hu, Wenzhong Zhou, and Ji Jung Kai. 2018. "Elemental Phase Partitioning in the γ-γ″ Ni2CoFeCrNb0.15 High Entropy Alloy" Entropy 20, no. 12: 910. https://doi.org/10.3390/e20120910