Extraction of the Proton and Electron Radii from Characteristic Atomic Lines and Entropy Principles
Abstract
:1. Introduction
2. Theory
- E1
- E2
- Constructive interference: There are constructive interferences among the X-rays and later on with the atomic nucleus or electron. This occurs only if ra ≈ rn + λ, where ra is the atomic radius and rn is the nucleus radius.
- E3
- E4
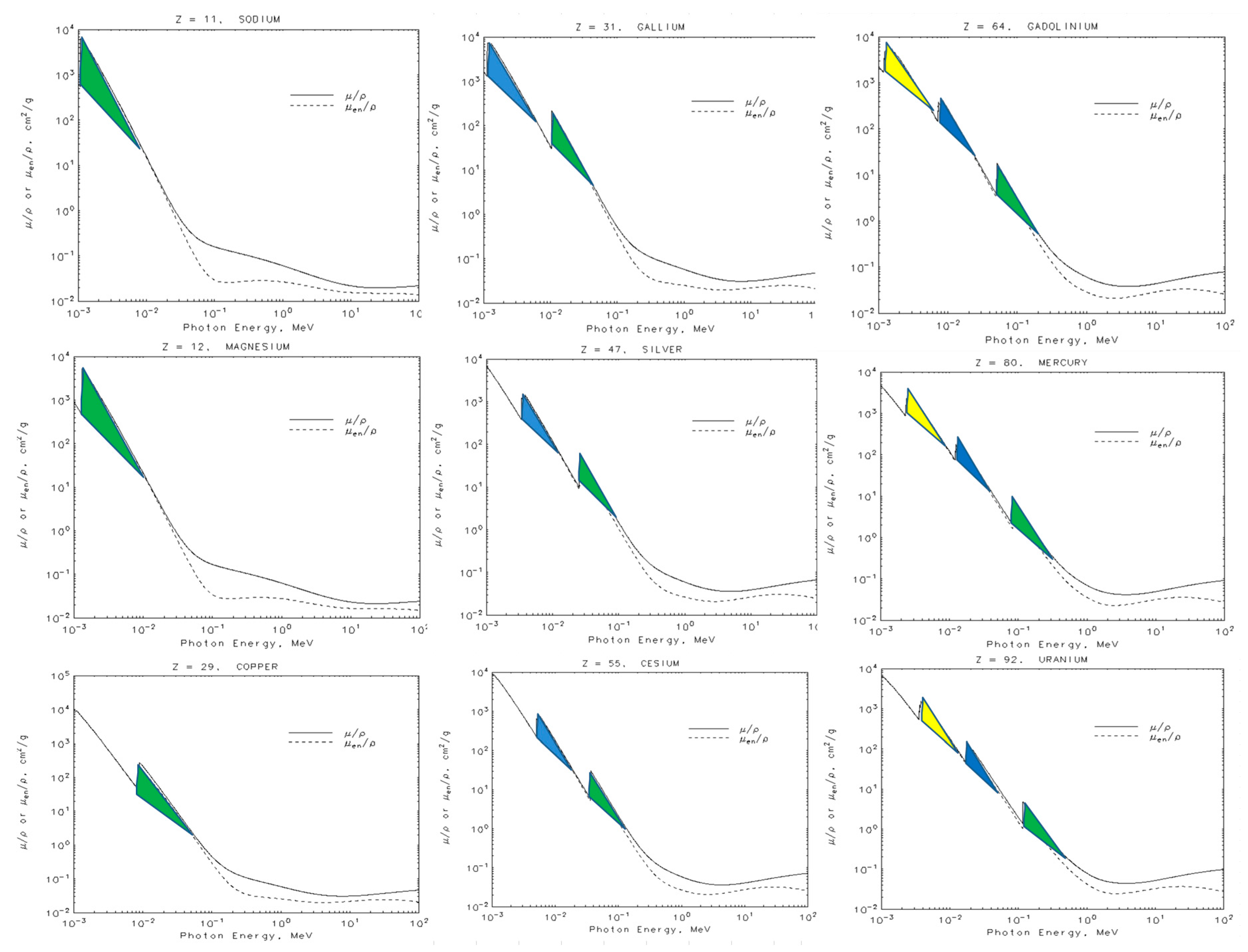
- The optimal cross section of the atomic nucleus depends on the proton radius rp. The proton radius will be measured indirectly through the atomic total cross section, which is a result of the interference between the nucleus (but always with a specific nucleon), X-rays and the electron [18].
3. Definitions
Cross Section
4. Resonance Region
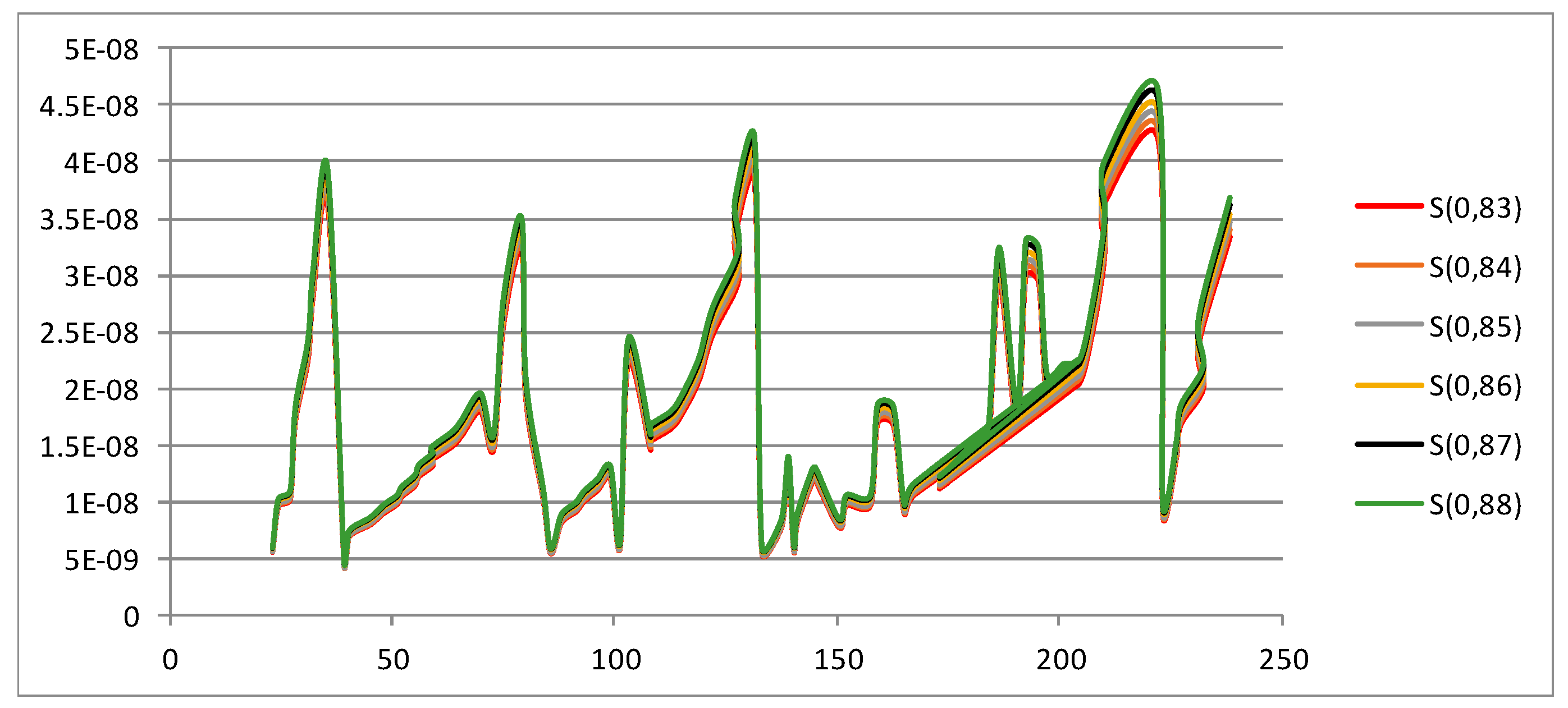
Shannon Entropy
5. Discussion of Results
- The cross section for the proton is dynamic. The proton is deformed and increases with the atomic weight A, due to nuclear force. Using the minimum entropy theory, we can calculate the optimal dimension of the proton radius and the conditions for the photon to be trapped in the resonance region corresponding to the K-shell. Once in this region, the photon interacts with the electron or with one of the nucleons. In this paper, we give a calculation method for the proton radius as a function of the resonance cross section. However, in a similar way, we can also obtain the electron radius, since the low energy X-rays are confined to the boundaries corresponding to electrons in the K-shell and protons in the nucleus.
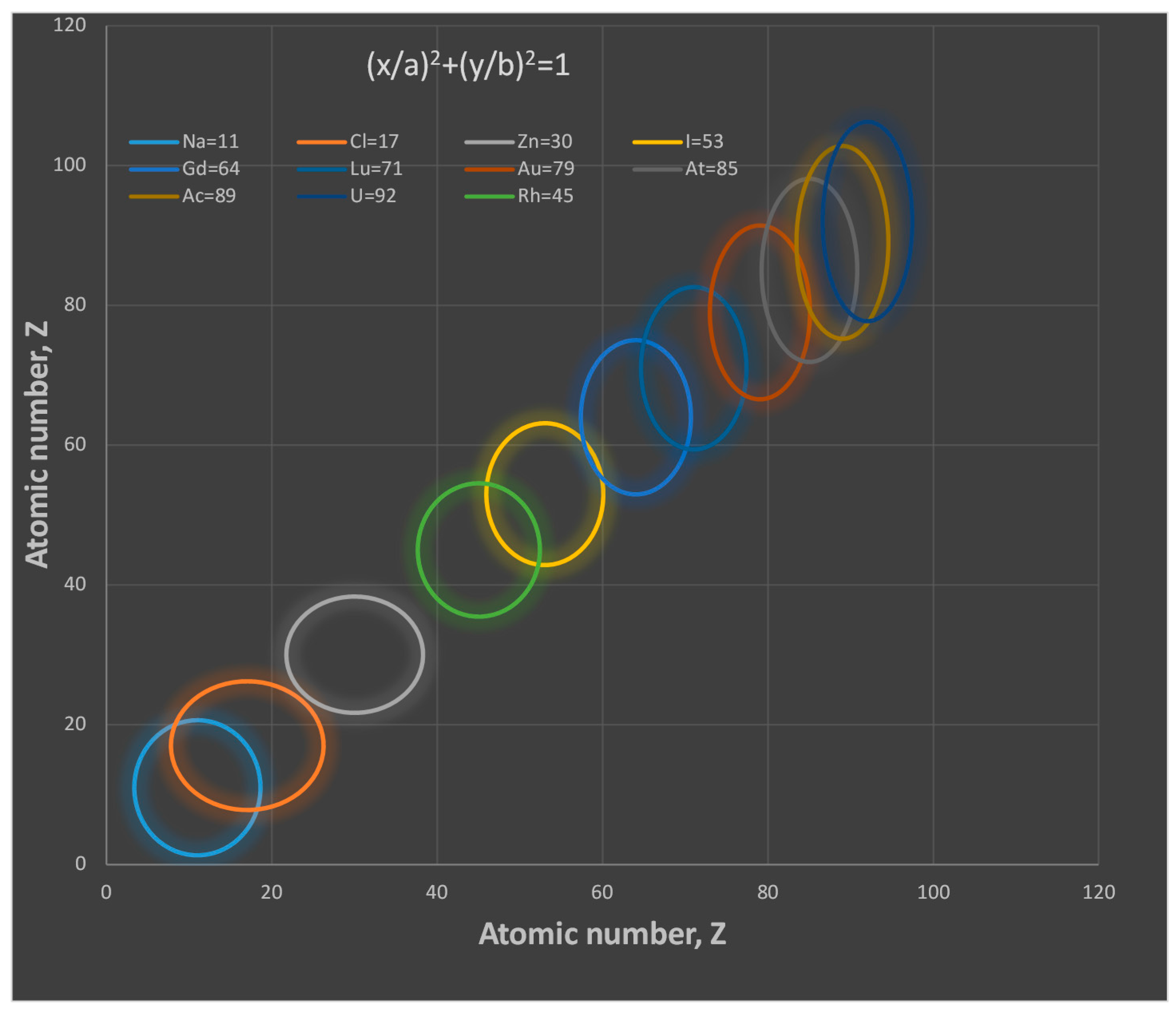
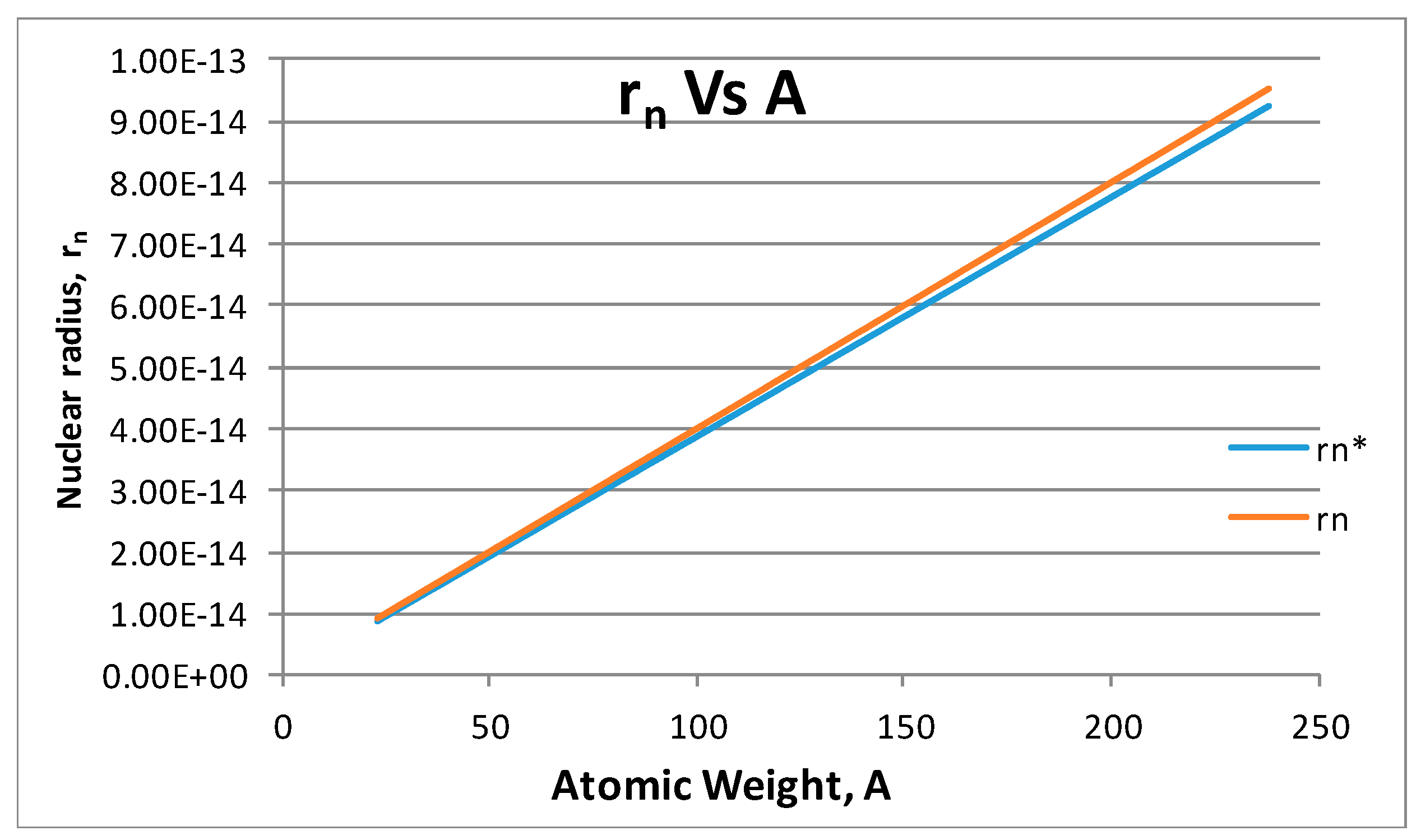
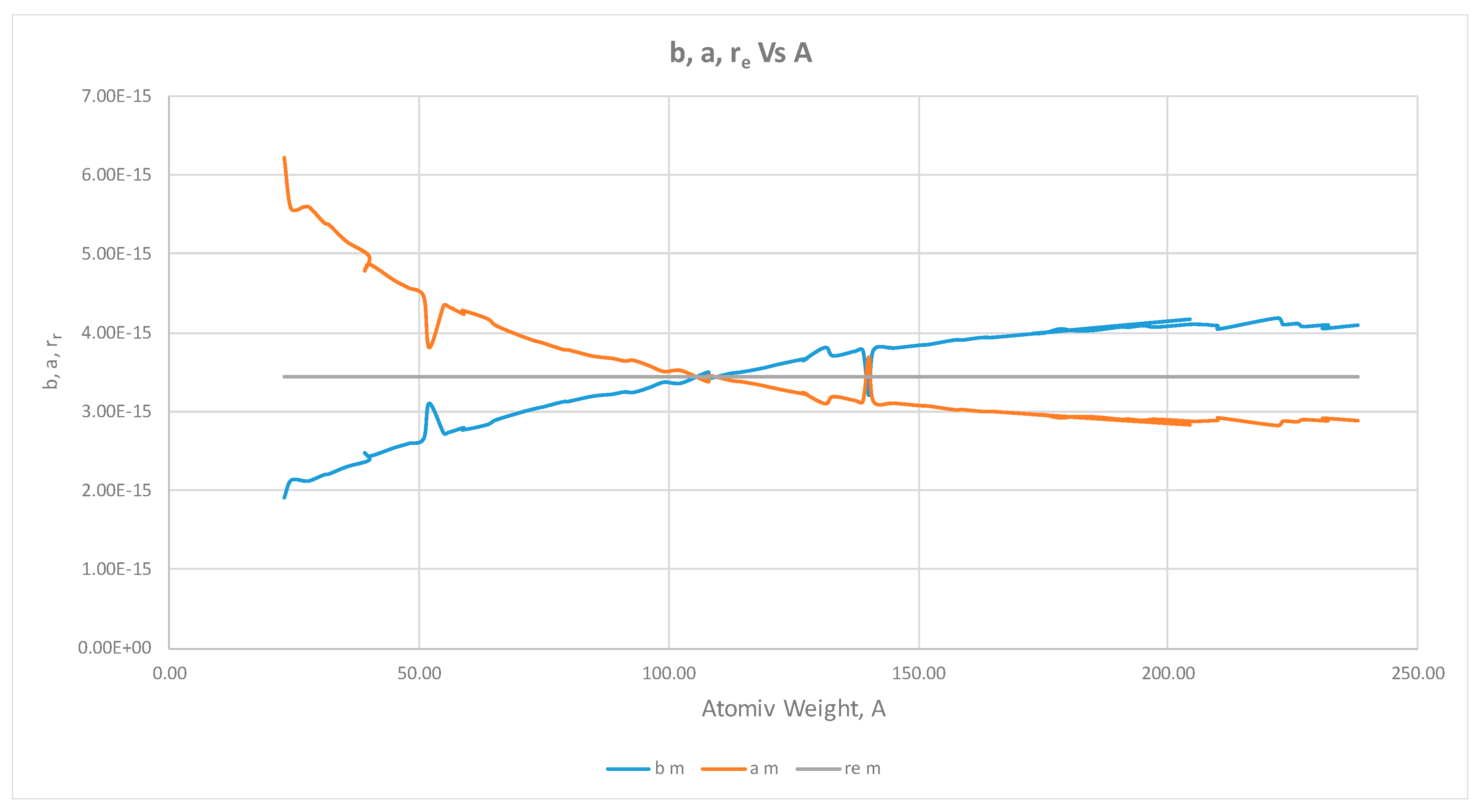
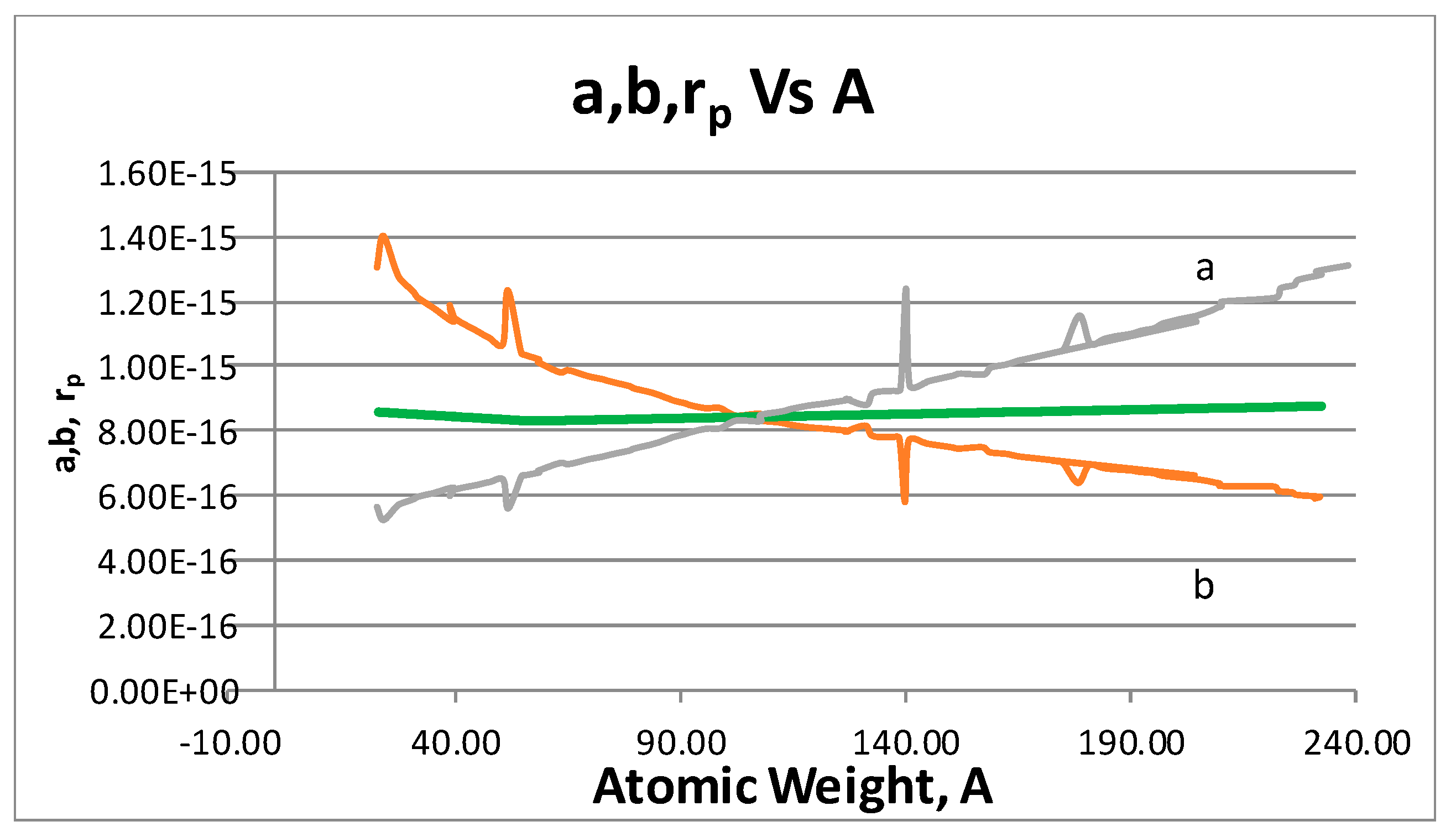
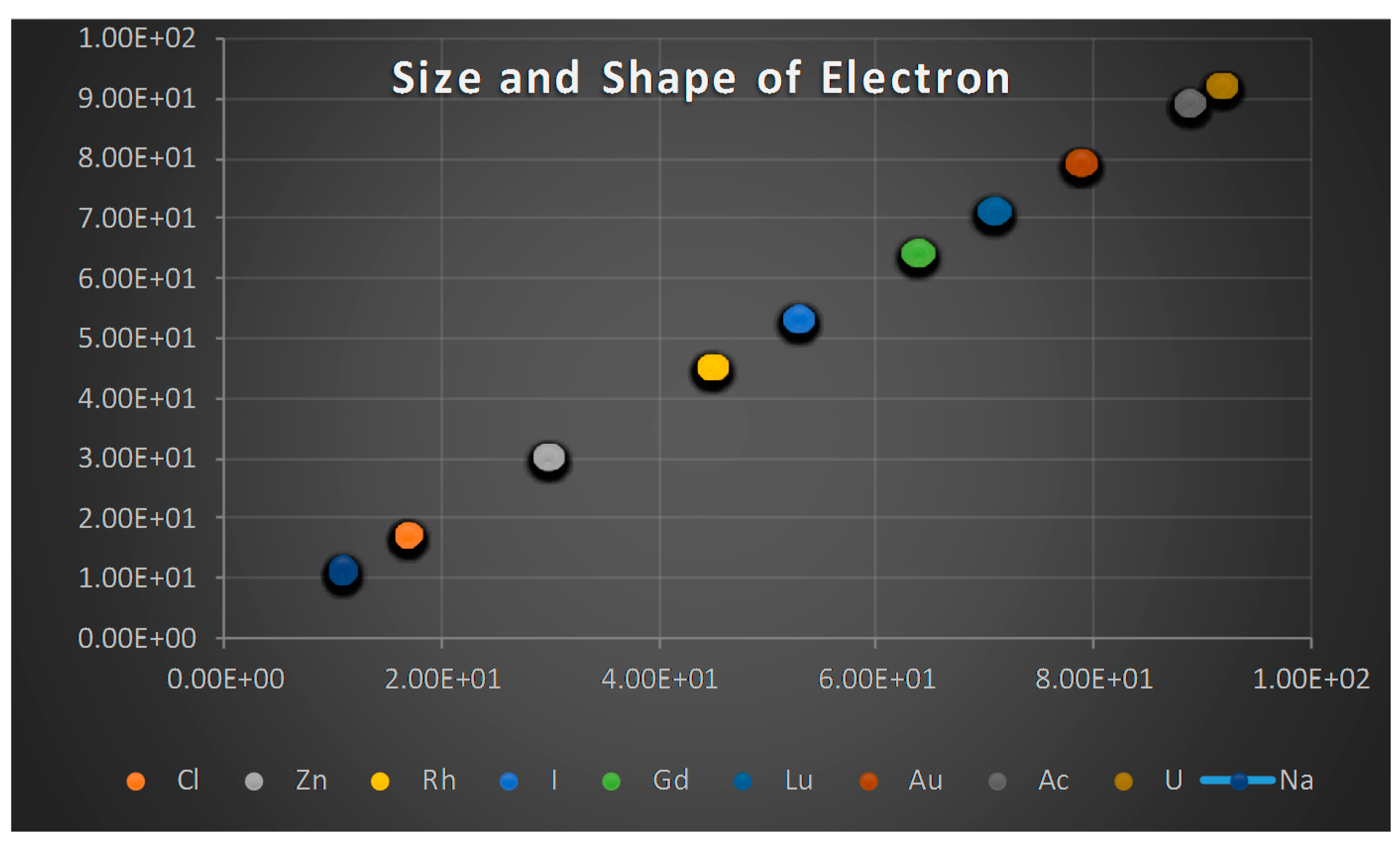
- The proton shape corresponds to a sphere with two different error measurements in length axis. From the experimental cross section, we can obtain two axes, the larger and the smaller. From the experiment we only obtain one axis, which is in the range of 1.3 fm ≤ b ≤ 0.59 fm, where 1.3 fm corresponds to sodium Z = 11 and 0.59 corresponds to Uranium Z = 92. We can calculate the second axis by using the formula rp2 = ab (Figure 3).
- Considering minimal deformation, the most probable value for the radius of the proton is obtained by averaging the measured value for one of its axes. The value obtained this way was 0.851 fm.
- This model allows us to explain the mass absorption curves for low energy X-rays and helps us to interpret the photoelectric effect in terms of the interference of wave properties. The question about why the K-shell has more probability than the more external ones, can be understood in terms of the effects of the nucleus in the resonance region, and the same for the electron. Moreover, the photons that come into the resonance region only have a small probability of producing a primary photoelectric effect, and for this reason, the secondary effect is predominant.
- 5.
Electron Results: Discussion and Analysis
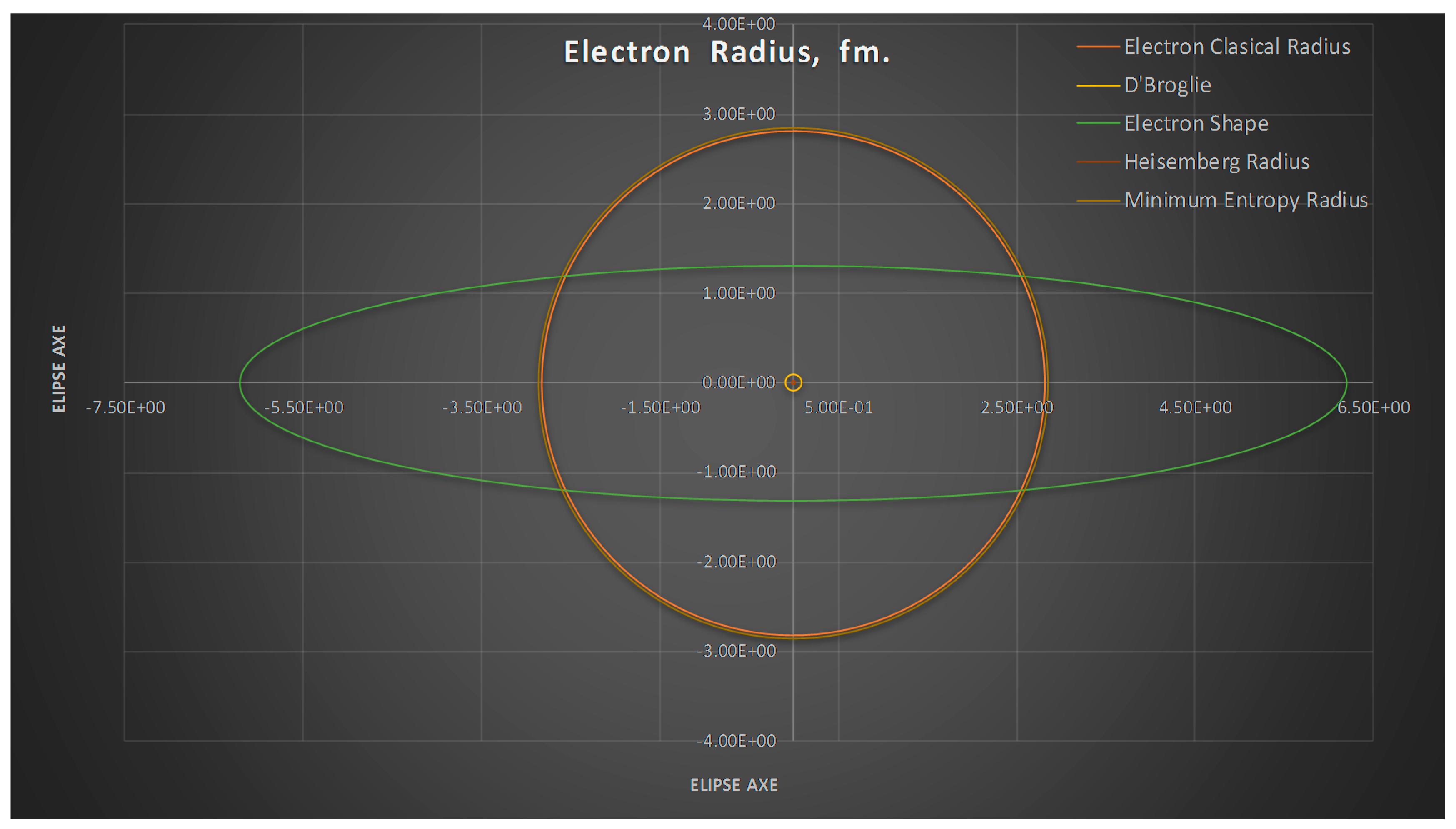
- The minimum entropy electron radius corresponds to the classical electron radius.
- 1A.
- The meaning of Theorem 3. The electron is not a perfect sphere. The volumetric ellipse axes vary as a function of the atom and measurement where the electron is located. However, the electron radius converges to a value called minimum entropy. Such value is the geometric mean of the two axes of the effective cross section re2 = ai bi.
- 1B.
- The second method to obtain the electron radius uses the same probability values that we found for the proton radius calculation. After using that information, we find the most probable value for the electron radius from Equations (33) and (34) and we get an experimental value in the range (2.75, 2.90) fm, Figure 7.
- 2.
- The interference of X-rays with the electron creates an effective resonance cross section. This last result depends on the electron radius, or any one of the ellipse axes, according to Equation (23). We have also analyzed the mass attenuation coefficients for elements with Z in the interval: 11 ≤ Z ≤ 92. The results are summarized in Table 1.
- We can see from Figure 7 the spherical electron radius together with its deformed cross section. Note that we still see the electron as sphere even if its cross section is deformed.
- 3.
- The uncertainty principle is applied in its most rigorous way, as follows:
- 4.
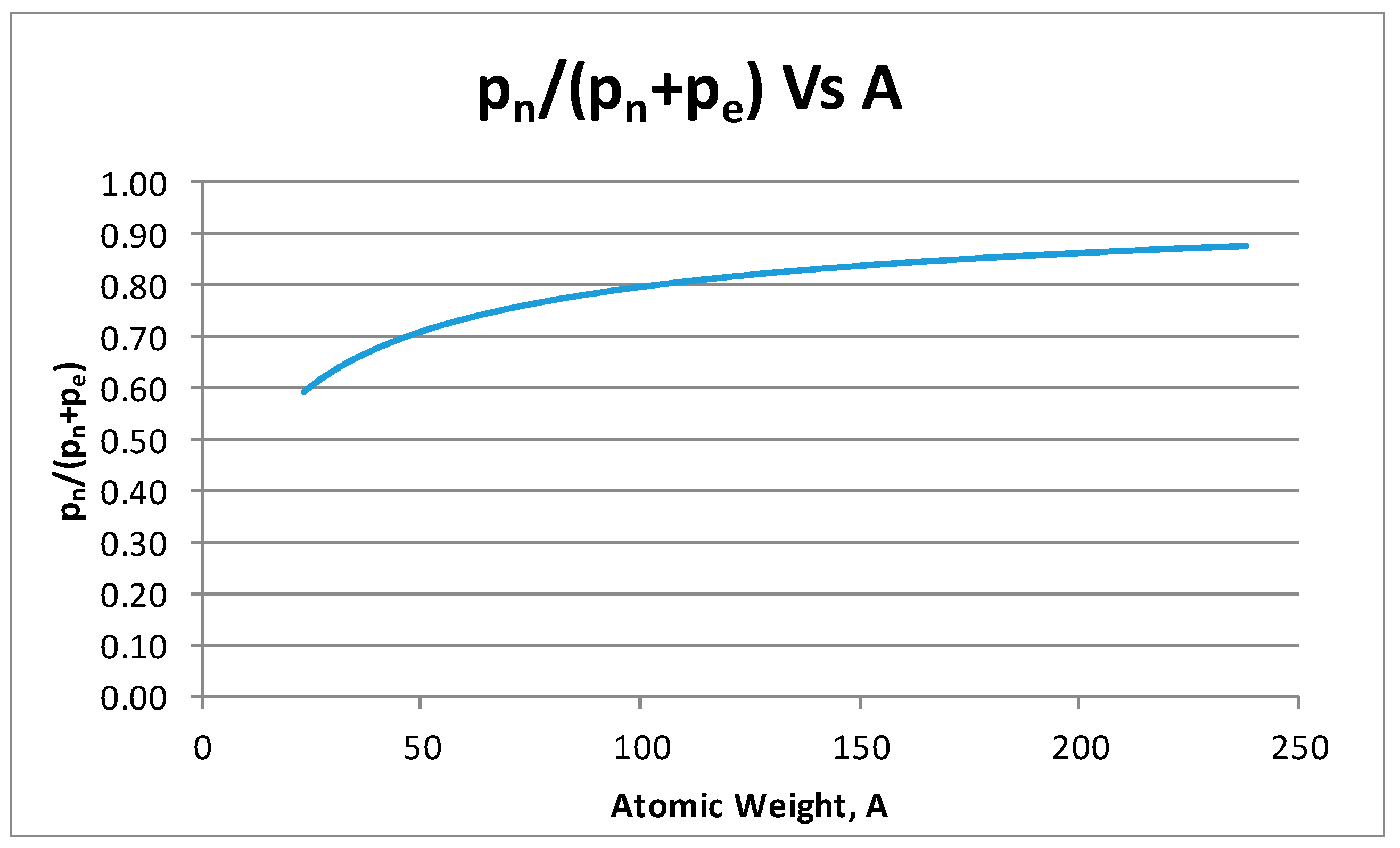
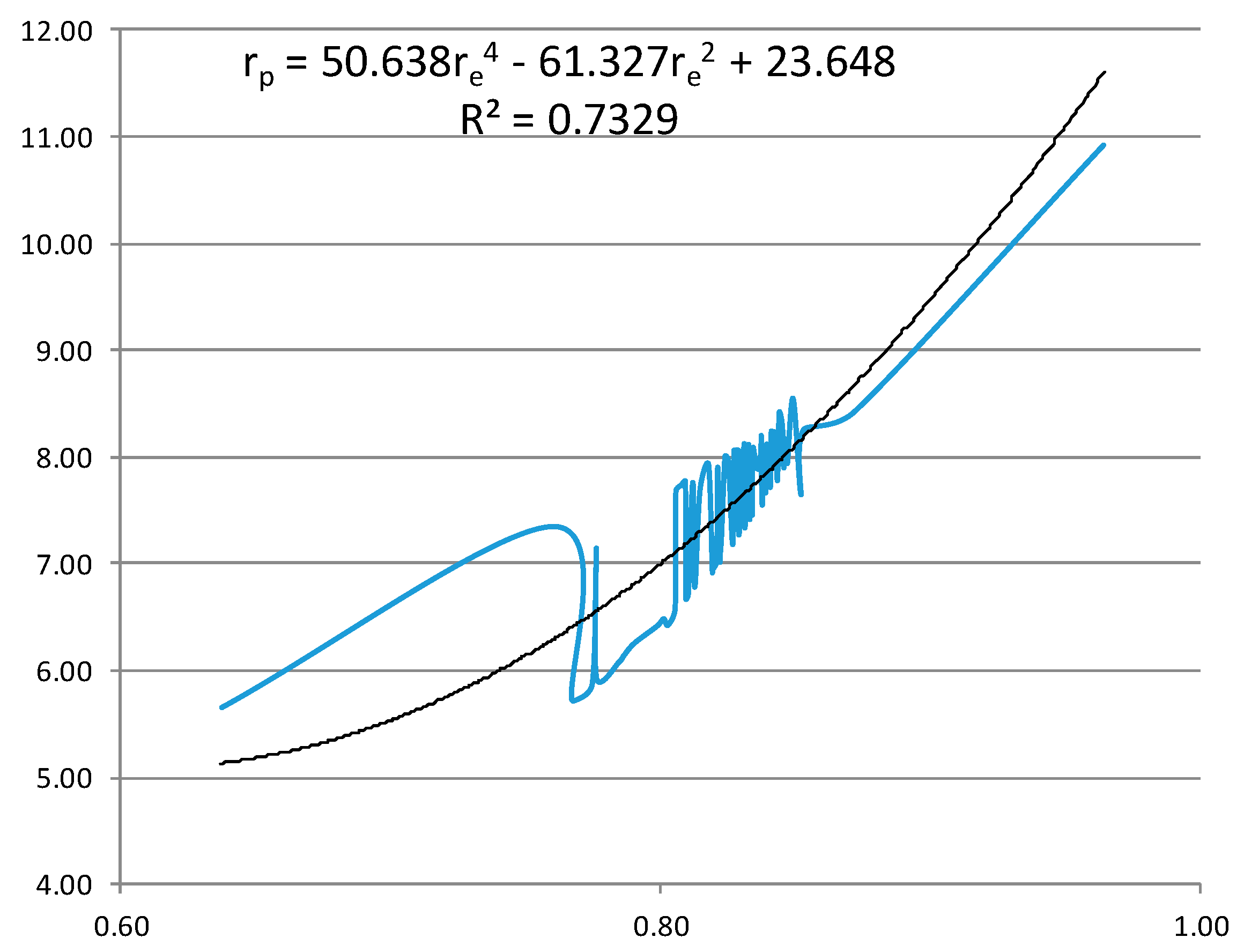
- From the experiment, the proton and electron radii have a functional relationship (Figure 11). It is found that the proton and electron radii are limited by the values of the two measured axes: (0.825 − 0341) fm < rp < (0.888 + 0.041) fm y (2.75 − 0.15) < re < (2.82 + 0.16) fm.
6. Methods
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Jentschura, U.D. Muonic bound systems, virtual particles, and proton radius. Phys. Rev. A 2015, 92, 012123. [Google Scholar] [CrossRef]
- Pohl, R.; Antognini, A.; Nez, F.; Amaro, F.D.; Biraben, F.; Cardoso, J.M.R.; Covita, D.S.; Dax, A.; Dhawan, S.; Fernandes, L.M.P.; et al. The size of the proton. Nature 2010, 466, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Strauch, S. Elastic Electron and Muon Scattering Experiment off the Proton at PSI. In Proceedings of the Particle and Nuclei International Conference 2014, Hamburg, Germany, 24–29 August 2014. [Google Scholar]
- Griffioen, K.; Carlson, C.; Maddox, S. Consistency of electron scattering data with a small proton radius. Phys. Rev. C 2016, 93, 065207. [Google Scholar] [CrossRef]
- Horbatsch, M.; Hessels, E.A.; Pineda, A. Proton radius from electron-scattering and chiral perturbation theory. Phys. Rev. C 2017, 95, 035203. [Google Scholar] [CrossRef]
- Kraus, E.; Mesick, K.E.; White, A.; Gilman, R.; Strauch, S. Polynomial fits and the proton radius puzzle. Phys. Rev. C 2014, 90, 045206. [Google Scholar] [CrossRef]
- Lee, G.; Arrington, J.R.; Hill, R.J. Extraction of the proton radius from electron-proton scattering data. Phys. Rev. D 2015, 92, 013013. [Google Scholar] [CrossRef]
- Olive, K.A. Review of Particle Physics (Particle Data Group). Chin. Phys. C 2014, 38, 090001. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. X-ray Mass Attenuation Coefficients, Radiation Division, PML, NIST, 2017. Available online: https://www.nist.gov/pml/x-ray-mass-attenuation-coefficients (accessed on 1 October 2016).
- Samuel, W. Introductory Nuclear Physics, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Mitchell, A.C.G.; Zemansky, M.W. Resonance Radiation and Exited Atoms; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Christopher, G. Quantum Information Theory and the Foundation of Quantum Mechanics. Ph.D. Thesis, Oxford University, London, UK, 2004. [Google Scholar]
- Max, B. Atomic Physics, 2nd ed.; Blackie & Son Limited: London, UK, 1937. [Google Scholar]
- David, J.J. Classic Electrodynamics; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Landau, L.D.; Lifschitz, E.M. Mechanics; Nauka Publishers: Moscow, Russia, 1988. [Google Scholar]
- Claude, C.T. Quantum Mechanics; Wiley-VCH: Hoboken, NJ, USA, 1992. [Google Scholar]
- Sick, I. On the rms-radius of the proton. Phys. Lett. B 2003, 576, 62–67. [Google Scholar] [CrossRef]
- Briggs, J.S.; Lane, A.M. The effect of atomic binding on a nuclear resonance. Phys. Lett. B 1981, 106, 436–438. [Google Scholar] [CrossRef]
- Blunden, P.G.; Sick, I. Proton radii and two-photon exchange. Phys. Rev. C 2005, 72, 057601. [Google Scholar] [CrossRef]
- Griffits, D. Introduction to Elementary Particles, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 2008. [Google Scholar]
- Karush, W. Isoperimetric Problems and Index Theorems in the Calculus of Variations. Ph.D. Thesis, Department of Mathematics, University of Chicago, Chicago, IL, USA, 1942. [Google Scholar]
- Fischer, M.; Kolachevsky, N.; Zimmermann, M.; Holzwarth, R.; Udem, T.; Hänsch, T.W.; Abgrall, M.; Grünert, J.; Maksimovic, I.; Bize, S.; et al. New limits on the drift of fundamental constants from laboratory measurements. Phys. Rev. Lett. 2004, 92, 230802. [Google Scholar] [CrossRef] [PubMed]
- Bliss, G.A. Lectures on the Calculus of Variations; University of Chicago Press: Chicago, IL, USA, 1946. [Google Scholar]
- Hestenes, M.R. Calculus of Variations and Optimal Control Theory; John Wiley & Sons: New York, NY, USA, 1966. [Google Scholar]
- Pars, L.A. An Introduction to the Calculus of Variations; John Wiley & Sons: New York, NY, USA, 1962. [Google Scholar]
- Karush, W. Mathematical Programming, Man-Computer Search and System Control; Technical Report SP-828; System Development Corporation: Santa Monica, CA, USA, 1962. [Google Scholar]
- Kuhn, H.W.; Tucker, A.W. Nonlinear programming. In Proceedings of the Second Berkeley Symposium on Mathematical Statistics and Probability, Statistical Laboratory of the University of California, Berkeley, CA, USA, 31 July–12 August 1950; Neyman, J., Ed.; University of California Press: Berkeley, CA, USA, 1951; pp. 481–492. [Google Scholar]

| ra | rp | E2 = E1 = E | λe = h/p | b | a | re | a/b | λ = hc/E | p = (E2/c2 + 2mE)1/2 | υ = (E/mc2 + 1) | β = v/c | (pe/(pn + pe)) | (σ2 − σ1)((pn + pe)/pe) | b | σ2 | σ1 | σ2 | σ1 | (σ2 − σ1)/σ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | m | MeV | m | m | m | m | m | Kg·m/s | m | m2 | m | cm2/gr | cm2/gr | cm2 | cm2 | % | |||
| 1.9 × 10−10 | 8.3 × 10−16 | 0.0010721 | 3.32187 × 10−16 | 7.4503 × 10−16 | 9.81203 × 10−16 | 2.67315 × 10−15 | 1.31699822 | 1.15802 × 10−9 | 1.99887 × 10−18 | 1.002098043 | 7.4503 × 10−16 | 0.38032585 | 5.91429 × 10−23 | 7.4503 × 10−16 | 6435 | 542.9 | 2.45661 × 10−19 | 2.07257 × 10−20 | 0.91563326 |
| 1.45 × 10−10 | 8.3 × 10−16 | 0.001305 | 3.01089 × 10−16 | 8.75431 × 10−16 | 8.35045 × 10−16 | 2.92396 × 10−15 | 0.95386719 | 9.51353 × 10−10 | 2.20533 × 10−18 | 1.002553816 | 8.75431 × 10−16 | 0.41438056 | 4.86109 × 10−23 | 8.75431 × 10−16 | 5444 | 453 | 2.19717 × 10−19 | 1.82828 × 10−20 | 0.91678913 |
| 1.431 × 10−10 | 8.3 × 10−16 | 0.0015596 | 2.75419 × 10−16 | 8.17537 × 10−16 | 8.94179 × 10−16 | 2.81511 × 10−15 | 1.0937471 | 7.96047 × 10−10 | 2.41087 × 10−18 | 1.003052055 | 8.17537 × 10−16 | 0.37957085 | 4.24312 × 10−23 | 8.17537 × 10−16 | 3957 | 362.1 | 1.77279 × 10−19 | 1.62226 × 10−20 | 0.90849128 |
| 1.11 × 10−10 | 8.3 × 10−16 | 0.00184 | 2.53566 × 10−16 | 8.00318 × 10−16 | 9.13418 × 10−16 | 2.78156 × 10−15 | 1.14131904 | 6.74737 × 10−10 | 2.61864 × 10−18 | 1.003600783 | 8.00318 × 10−16 | 0.36766952 | 3.65753 × 10−23 | 8.00318 × 10−16 | 3190 | 309 | 1.48796 × 10−19 | 1.44132 × 10−20 | 0.9031348 |
| 9.8 × 10−11 | 8.3 × 10−16 | 0.00215 | 2.34575 × 10−16 | 8.0335 × 10−16 | 9.09971 × 10−16 | 2.7877 × 10−15 | 1.13272048 | 5.77449 × 10−10 | 2.83065 × 10−18 | 1.004207436 | 8.0335 × 10−16 | 0.35368271 | 3.23379 × 10−23 | 8.0335 × 10−16 | 2470 | 249 | 1.27025 × 10−19 | 1.28053 × 10−20 | 0.89919028 |
| 8.8 × 10−11 | 8.3 × 10−16 | 0.00247 | 2.18853 × 10−16 | 7.96621 × 10−16 | 9.17658 × 10−16 | 2.77099 × 10−15 | 1.15193793 | 5.02638 × 10−10 | 3.034 × 10−18 | 1.004833659 | 7.96621 × 10−16 | 0.34568665 | 2.85404 × 10−23 | 7.96621 × 10−16 | 2070 | 217 | 1.10214 × 10−19 | 1.15539 × 10−20 | 0.89516908 |
| 7.1 × 10−11 | 8.3 × 10−16 | 0.002822 | 2.04749 × 10−16 | 8.06047 × 10−16 | 9.06926 × 10−16 | 2.78582 × 10−15 | 1.12515151 | 4.39942 × 10−10 | 3.24299 × 10−18 | 1.005522505 | 8.06047 × 10−16 | 0.33306326 | 2.58065 × 10−23 | 8.06047 × 10−16 | 1640 | 177 | 9.65487 × 10−20 | 1.04202 × 10−20 | 0.89207317 |
| 7.9 × 10−11 | 8.3 × 10−16 | 0.0032029 | 1.92189 × 10−16 | 8.0681 × 10−16 | 9.06069 × 10−16 | 2.77356 × 10−15 | 1.12302669 | 3.87622 × 10−10 | 3.45493 × 10−18 | 1.006267906 | 8.0681 × 10−16 | 0.3368301 | 2.23054 × 10−23 | 8.0681 × 10−16 | 1275 | 142.7 | 8.45775 × 10−20 | 9.46604 × 10−21 | 0.88807843 |
| 2.43 × 10−10 | 8.3 × 10−16 | 0.0036074 | 1.81094 × 10−16 | 8.61142 × 10−16 | 8.48902 × 10−16 | 2.90268 × 10−15 | 0.98578609 | 3.44158 × 10−10 | 3.66661 × 10−18 | 1.007059491 | 8.61142 × 10−16 | 0.3368301 | 2.05867 × 10−23 | 8.61142 × 10−16 | 1200 | 133 | 7.79125 × 10−20 | 8.63531 × 10−21 | 0.88916667 |
| 1.94 × 10−10 | 8.3 × 10−16 | 0.0040381 | 1.71164 × 10−16 | 8.26435 × 10−16 | 8.84552 × 10−16 | 2.82948 × 10−15 | 1.070322 | 3.0745 × 10−10 | 3.87932 × 10−18 | 1.007902348 | 8.26435 × 10−16 | 0.32190293 | 1.87112 × 10−23 | 8.26435 × 10−16 | 1023 | 118 | 6.80852 × 10−20 | 7.85343 × 10−21 | 0.88465298 |
| 1.84 × 10−10 | 8.3 × 10−16 | 0.00449 | 1.62322 × 10−16 | 8.31531 × 10−16 | 8.79132 × 10−16 | 2.83979 × 10−15 | 1.05724525 | 2.76507 × 10−10 | 4.09063 × 10−18 | 1.008786693 | 8.31531 × 10−16 | 0.30696061 | 1.74654 × 10−23 | 8.31531 × 10−16 | 815 | 96.9 | 6.08462 × 10−20 | 7.23435 × 10−21 | 0.88110429 |
| 1.76 × 10−10 | 8.3 × 10−16 | 0.00497 | 1.54285 × 10−16 | 8.31466 × 10−16 | 8.792 × 10−16 | 2.83908 × 10−15 | 1.05741018 | 2.49802 × 10−10 | 4.30374 × 10−18 | 1.009726027 | 8.31466 × 10−16 | 0.29800529 | 1.61198 × 10−23 | 8.31466 × 10−16 | 688 | 83.8 | 5.47006 × 10−20 | 6.66266 × 10−21 | 0.87819767 |
| 1.71 × 10−10 | 8.3 × 10−16 | 0.0054651 | 1.4713 × 10−16 | 8.36832 × 10−16 | 8.73562 × 10−16 | 2.85004 × 10−15 | 1.04389139 | 2.27172 × 10−10 | 4.51301 × 10−18 | 1.010694912 | 8.36832 × 10−16 | 0.29102595 | 1.49463 × 10−23 | 8.36832 × 10−16 | 587 | 72.77 | 4.96531 × 10−20 | 6.15546 × 10−21 | 0.87603066 |
| 1.66 × 10−10 | 8.3 × 10−16 | 0.0059892 | 1.40545 × 10−16 | 1.06767 × 10−15 | 6.84695 × 10−16 | 3.30564 × 10−15 | 0.64130057 | 2.07292 × 10−10 | 4.72446 × 10−18 | 1.011720548 | 1.06767 × 10−15 | 0.35264878 | 1.30228 × 10−23 | 1.06767 × 10−15 | 597.7 | 65.74 | 5.16003 × 10−20 | 5.67543 × 10−21 | 0.89001171 |
| 1.61 × 10−10 | 8.3 × 10−16 | 0.00654 | 1.34497 × 10−16 | 8.35959 × 10−16 | 8.74475 × 10−16 | 2.84669 × 10−15 | 1.04607322 | 1.89834 × 10−10 | 4.93692 × 10−18 | 1.012798434 | 8.35959 × 10−16 | 0.28026267 | 1.28253 × 10−23 | 8.35959 × 10−16 | 452 | 58 | 4.1236 × 10−20 | 5.29134 × 10−21 | 0.87168142 |
| 1.558 × 10−10 | 8.3 × 10−16 | 0.00711 | 1.28993 × 10−16 | 8.32631 × 10−16 | 8.7797 × 10−16 | 2.83848 × 10−15 | 1.054452 | 1.74615 × 10−10 | 5.14757 × 10−18 | 1.013913894 | 8.32631 × 10−16 | 0.27690045 | 1.18832 × 10−23 | 8.32631 × 10−16 | 408 | 53.2 | 3.78384 × 10−20 | 4.93383 × 10−21 | 0.86960784 |
| 1.52 × 10−10 | 8.3 × 10−16 | 0.00771 | 1.23872 × 10−16 | 8.37916 × 10−16 | 8.72432 × 10−16 | 2.84929 × 10−15 | 1.04119234 | 1.61027 × 10−10 | 5.36037 × 10−18 | 1.015088063 | 8.37916 × 10−16 | 0.27129321 | 1.1142 × 10−23 | 8.37916 × 10−16 | 356 | 47.1 | 3.48366 × 10−20 | 4.609 × 10−21 | 0.86769663 |
| 1.49 × 10−10 | 8.3 × 10−16 | 0.00833 | 1.19173 × 10−16 | 8.2619 × 10−16 | 8.84815 × 10−16 | 2.82221 × 10−15 | 1.07095852 | 1.49041 × 10−10 | 5.57173 × 10−18 | 1.01630137 | 8.2619 × 10−16 | 0.2680679 | 1.03504 × 10−23 | 8.2619 × 10−16 | 329 | 44.3 | 3.20634 × 10−20 | 4.31735 × 10−21 | 0.86534954 |
| 1.45 × 10−10 | 8.3 × 10−16 | 0.0089789 | 1.14786 × 10−16 | 8.20536 × 10−16 | 8.90912 × 10−16 | 2.81146 × 10−15 | 1.08576779 | 1.3827 × 10−10 | 5.78468 × 10−18 | 1.017571233 | 8.20536 × 10−16 | 0.25633201 | 9.88449 × 10−24 | 8.20536 × 10−16 | 278 | 38.3 | 2.93366 × 10−20 | 4.0417 × 10−21 | 0.86223022 |
| 1.42 × 10−10 | 8.3 × 10−16 | 0.00966 | 1.10665 × 10−16 | 8.38682 × 10−16 | 8.71635 × 10−16 | 2.84463 × 10−15 | 1.03929089 | 1.28521 × 10−10 | 6.00007 × 10−18 | 1.01890411 | 8.38682 × 10−16 | 0.25714096 | 9.22926 × 10−24 | 8.38682 × 10−16 | 254 | 35.1 | 2.7588 × 10−20 | 3.81236 × 10−21 | 0.86181102 |
| 1.35 × 10−10 | 8.3 × 10−16 | 0.0103671 | 1.06825 × 10−16 | 8.44395 × 10−16 | 8.65739 × 10−16 | 2.86404 × 10−15 | 1.02527748 | 1.19755 × 10−10 | 6.21579 × 10−18 | 1.020287867 | 8.44395 × 10−16 | 0.25164879 | 8.75949 × 10−24 | 8.44395 × 10−16 | 221 | 31 | 2.55858 × 10−20 | 3.58896 × 10−21 | 0.85972851 |
| 1.52 × 10−10 | 8.3 × 10−16 | 0.0111 | 1.03238 × 10−16 | 8.49084 × 10−16 | 8.60957 × 10−16 | 2.87074 × 10−15 | 1.01398278 | 1.11848 × 10−10 | 6.43175 × 10−18 | 1.021722114 | 8.49084 × 10−16 | 0.24740235 | 8.28839 × 10−24 | 8.49084 × 10−16 | 198 | 28.1 | 2.38831 × 10−20 | 3.38947 × 10−21 | 0.85808081 |
| 1.14 × 10−10 | 8.3 × 10−16 | 0.0118667 | 9.98471 × 10−17 | 8.49827 × 10−16 | 8.60205 × 10−16 | 2.87015 × 10−15 | 1.01221185 | 1.04622 × 10−10 | 6.65017 × 10−18 | 1.023222505 | 8.49827 × 10−16 | 0.24351097 | 7.83861 × 10−24 | 8.49827 × 10−16 | 179.2 | 25.77 | 2.22939 × 10−20 | 3.20599 × 10−21 | 0.8561942 |
| 1.03 × 10−10 | 8.3 × 10−16 | 0.0126578 | 9.66766 × 10−17 | 8.54015 × 10−16 | 8.55986 × 10−16 | 2.87873 × 10−15 | 1.00230776 | 9.8083 × 10−11 | 6.86826 × 10−18 | 1.024770646 | 8.54015 × 10−16 | 0.23819988 | 7.47066 × 10−24 | 8.54015 × 10−16 | 158.9 | 23.18 | 2.08344 × 10−20 | 3.03927 × 10−21 | 0.85412209 |
| 1.35 × 10−10 | 8.3 × 10−16 | 0.0134737 | 9.37038 × 10−17 | 8.49723 × 10−16 | 8.6031 × 10−16 | 2.8675 × 10−15 | 1.01245862 | 9.21436 × 10−11 | 7.08616 × 10−18 | 1.026367319 | 8.49723 × 10−16 | 0.23536126 | 7.06563 × 10−24 | 8.49723 × 10−16 | 147.1 | 21.76 | 1.95168 × 10−20 | 2.88706 × 10−21 | 0.85207342 |
| 1.9 × 10−10 | 8.3 × 10−16 | 0.0143256 | 9.08749 × 10−17 | 8.51054 × 10−16 | 8.58964 × 10−16 | 2.86937 × 10−15 | 1.00929502 | 8.66641 × 10−11 | 7.30674 × 10−18 | 1.028034442 | 8.51054 × 10−16 | 0.22992348 | 6.7536 × 10−24 | 8.51054 × 10−16 | 131.3 | 19.71 | 1.82708 × 10−20 | 2.74271 × 10−21 | 0.84988576 |
| 2.65 × 10−10 | 8.3 × 10−16 | 0.015199 | 8.82253 × 10−17 | 8.48897 × 10−16 | 8.61147 × 10−16 | 2.86291 × 10−15 | 1.01443044 | 8.1684 × 10−11 | 7.52619 × 10−18 | 1.02974364 | 8.48897 × 10−16 | 0.22681006 | 6.42019 × 10−24 | 8.48897 × 10−16 | 121 | 18.4 | 1.71731 × 10−20 | 2.61145 × 10−21 | 0.84793388 |
| 2.19 × 10−10 | 8.3 × 10−16 | 0.0161046 | 8.57088 × 10−17 | 8.43835 × 10−16 | 8.66313 × 10−16 | 2.84958 × 10−15 | 1.0266387 | 7.70907 × 10−11 | 7.74716 × 10−18 | 1.031515851 | 8.43835 × 10−16 | 0.22230144 | 6.13006 × 10−24 | 8.43835 × 10−16 | 110.8 | 17.14 | 1.6121 × 10−20 | 2.49381 × 10−21 | 0.84530686 |
| 2.12 × 10−10 | 8.3 × 10−16 | 0.0170384 | 8.33271 × 10−17 | 8.40366 × 10−16 | 8.69888 × 10−16 | 2.83978 × 10−15 | 1.03513002 | 7.28657 × 10−11 | 7.9686 × 10−18 | 1.033343249 | 8.40366 × 10−16 | 0.21943832 | 5.83858 × 10−24 | 8.40366 × 10−16 | 102.9 | 16.12 | 1.5192 × 10−20 | 2.37993 × 10−21 | 0.84334305 |
| 2.06 × 10−10 | 8.3 × 10−16 | 0.017997 | 8.10775 × 10−17 | 8.40175 × 10−16 | 8.70087 × 10−16 | 2.83762 × 10−15 | 1.03560254 | 6.89846 × 10−11 | 8.18969 × 10−18 | 1.035219178 | 8.40175 × 10−16 | 0.21626625 | 5.58225 × 10−24 | 8.40175 × 10−16 | 94.7 | 15 | 1.43446 × 10−20 | 2.27212 × 10−21 | 0.84160507 |
| 1.98 × 10−10 | 8.3 × 10−16 | 0.0189856 | 7.89384 × 10−17 | 8.3501 × 10−16 | 8.75469 × 10−16 | 2.81176 × 10−15 | 1.04845334 | 6.53925 × 10−11 | 8.41162 × 10−18 | 1.037153816 | 8.3501 × 10−16 | 0.21113252 | 5.35698 × 10−24 | 8.3501 × 10−16 | 87.84 | 14.09 | 1.3552 × 10−20 | 2.17382 × 10−21 | 0.83959472 |
| 1.9 × 10−10 | 8.3 × 10−16 | 0.019999 | 7.69124 × 10−17 | 8.38586 × 10−16 | 8.71735 × 10−16 | 2.82877 × 10−15 | 1.03952958 | 6.20789 × 10−11 | 8.6332 × 10−18 | 1.039136986 | 8.38586 × 10−16 | 0.20958319 | 5.12713 × 10−24 | 8.38586 × 10−16 | 80.6 | 13.1 | 1.28406 × 10−20 | 2.08699 × 10−21 | 0.83746898 |
| 1.83 × 10−10 | 8.3 × 10−16 | 0.021044 | 7.49784 × 10−17 | 8.51277 × 10−16 | 8.58739 × 10−16 | 2.85851 × 10−15 | 1.00876587 | 5.89962 × 10−11 | 8.85588 × 10−18 | 1.041181996 | 8.51277 × 10−16 | 0.20968537 | 4.89693 × 10−24 | 8.51277 × 10−16 | 74.81 | 12.29 | 1.22866 × 10−20 | 2.01848 × 10−21 | 0.83571715 |
| 2.65 × 10−10 | 8.3 × 10−16 | 0.0221 | 7.31652 × 10−17 | 8.38045 × 10−16 | 8.72298 × 10−16 | 2.8237 × 10−15 | 1.04087264 | 5.61772 × 10−11 | 9.07536 × 10−18 | 1.043248532 | 8.38045 × 10−16 | 0.20330645 | 4.73511 × 10−24 | 8.38045 × 10−16 | 68.8 | 11.4 | 1.15468 × 10−20 | 1.91327 × 10−21 | 0.83430233 |
| 1.34 × 10−10 | 8.3 × 10−16 | 0.0232 | 7.14096 × 10−17 | 8.33355 × 10−16 | 8.77207 × 10−16 | 2.81177 × 10−15 | 1.05262153 | 5.35136 × 10−11 | 9.29847 × 10−18 | 1.045401174 | 8.33355 × 10−16 | 0.20000646 | 4.55397 × 10−24 | 8.33355 × 10−16 | 64.1 | 10.8 | 1.09538 × 10−20 | 1.84557 × 10−21 | 0.83151326 |
| 1.69 × 10−10 | 8.3 × 10−16 | 0.0244 | 6.96315 × 10−17 | 8.60007 × 10−16 | 8.50022 × 10−16 | 2.87351 × 10−15 | 0.98838955 | 5.08818 × 10−11 | 9.53592 × 10−18 | 1.047749511 | 8.60007 × 10−16 | 0.20194504 | 4.34616 × 10−24 | 8.60007 × 10−16 | 59 | 10 | 1.05681 × 10−20 | 1.7912 × 10−21 | 0.83050847 |
| 1.65 × 10−10 | 8.3 × 10−16 | 0.025514 | 6.80944 × 10−17 | 8.33019 × 10−16 | 8.77561 × 10−16 | 2.80655 × 10−15 | 1.05346946 | 4.86602 × 10−11 | 9.75117 × 10−18 | 1.04992955 | 8.33019 × 10−16 | 0.19442131 | 4.22078 × 10−24 | 8.33019 × 10−16 | 55.39 | 9.59 | 9.92435 × 10−21 | 1.71826 × 10−21 | 0.82686405 |
| 1.61 × 10−10 | 8.3 × 10−16 | 0.0267 | 6.65648 × 10−17 | 8.3772 × 10−16 | 8.72636 × 10−16 | 2.8157 × 10−15 | 1.04168041 | 4.64987 × 10−11 | 9.97523 × 10−18 | 1.052250489 | 8.3772 × 10−16 | 0.19118457 | 4.08514 × 10−24 | 8.3772 × 10−16 | 50.65 | 8.809 | 9.45448 × 10−21 | 1.64431 × 10−21 | 0.82608095 |
| 1.56 × 10−10 | 8.3 × 10−16 | 0.0279 | 6.51176 × 10−17 | 8.3565 × 10−16 | 8.74799 × 10−16 | 2.80837 × 10−15 | 1.04684853 | 4.44988 × 10−11 | 1.01969 × 10−17 | 1.054598826 | 8.3565 × 10−16 | 0.18822821 | 3.94774 × 10−24 | 8.3565 × 10−16 | 47.3 | 8.32 | 9.0168 × 10−21 | 1.58604 × 10−21 | 0.82410148 |
| 1.45 × 10−10 | 8.3 × 10−16 | 0.0292001 | 6.36515 × 10−17 | 8.37622 × 10−16 | 8.72738 × 10−16 | 2.81069 × 10−15 | 1.04192371 | 4.25175 × 10−11 | 1.04318 × 10−17 | 1.057143053 | 8.37622 × 10−16 | 0.18508991 | 3.817 × 10−24 | 8.37622 × 10−16 | 43.6 | 7.76 | 8.59455 × 10−21 | 1.52967 × 10−21 | 0.82201835 |
| 1.33 × 10−10 | 8.3 × 10−16 | 0.030491 | 6.22895 × 10−17 | 8.42372 × 10−16 | 8.67817 × 10−16 | 2.81987 × 10−15 | 1.030206 | 4.07174 × 10−11 | 1.06599 × 10−17 | 1.059669276 | 8.42372 × 10−16 | 0.18349443 | 3.68367 × 10−24 | 8.42372 × 10−16 | 40.73 | 7.31 | 8.2378 × 10−21 | 1.47848 × 10−21 | 0.82052541 |
| 1.23 × 10−10 | 8.3 × 10−16 | 0.0318 | 6.0994 × 10−17 | 8.4856 × 10−16 | 8.61489 × 10−16 | 2.8326 × 10−15 | 1.01523657 | 3.90414 × 10−11 | 1.08863 × 10−17 | 1.06223092 | 8.4856 × 10−16 | 0.18022094 | 3.58117 × 10−24 | 8.4856 × 10−16 | 37.2 | 6.74 | 7.88212 × 10−21 | 1.4281 × 10−21 | 0.8188172 |
| 1.15 × 10−10 | 8.3 × 10−16 | 0.0331694 | 5.97216 × 10−17 | 8.4295 × 10−16 | 8.67222 × 10−16 | 2.81478 × 10−15 | 1.02879346 | 3.74295 × 10−11 | 1.11182 × 10−17 | 1.064910763 | 8.4295 × 10−16 | 0.17890145 | 3.44527 × 10−24 | 8.4295 × 10−16 | 35.82 | 6.55 | 7.54808 × 10−21 | 1.38023 × 10−21 | 0.81714126 |
| 1.08 × 10−10 | 8.3 × 10−16 | 0.0356 | 5.76468 × 10−17 | 8.81889 × 10−16 | 8.28931 × 10−16 | 2.90333 × 10−15 | 0.93994974 | 3.4874 × 10−11 | 1.15184 × 10−17 | 1.069667319 | 8.81889 × 10−16 | 0.18473575 | 3.19014 × 10−24 | 8.81889 × 10−16 | 33.2 | 6.13 | 7.23856 × 10−21 | 1.33652 × 10−21 | 0.81536145 |
| 2.98 × 10−10 | 8.3 × 10−16 | 0.0359 | 5.74055 × 10−17 | 8.41564 × 10−16 | 8.68651 × 10−16 | 2.80999 × 10−15 | 1.03218615 | 3.45826 × 10−11 | 1.15668 × 10−17 | 1.070254403 | 8.41564 × 10−16 | 0.17393222 | 3.24433 × 10−24 | 8.41564 × 10−16 | 31.4 | 5.86 | 6.92953 × 10−21 | 1.29322 × 10−21 | 0.8133758 |
| 2.53 × 10−10 | 8.3 × 10−16 | 0.0374 | 5.62425 × 10−17 | 8.47454 × 10−16 | 8.62614 × 10−16 | 2.81925 × 10−15 | 1.01788892 | 3.31956 × 10−11 | 1.1806 × 10−17 | 1.073189824 | 8.47454 × 10−16 | 0.17176851 | 3.14575 × 10−24 | 8.47454 × 10−16 | 29.2 | 5.5 | 6.65737 × 10−21 | 1.25396 × 10−21 | 0.81164384 |
| 1.95 × 10−10 | 8.3 × 10−16 | 0.0389 | 5.51475 × 10−17 | 8.45215 × 10−16 | 8.64899 × 10−16 | 2.81083 × 10−15 | 1.0232885 | 3.19156 × 10−11 | 1.20404 × 10−17 | 1.076125245 | 8.45215 × 10−16 | 0.16982759 | 3.0463 × 10−24 | 8.45215 × 10−16 | 27.7 | 5.27 | 6.38898 × 10−21 | 1.21552 × 10−21 | 0.80974729 |
| 2.98 × 10−10 | 8.3 × 10−16 | 0.03598 | 5.40873 × 10−17 | 6.0614 × 10−16 | 1.20603 × 10−15 | 2.37693 × 10−15 | 1.98969344 | 3.07002 × 10−11 | 1.22765 × 10−17 | 1.079138943 | 6.0614 × 10−16 | 0.12698018 | 3.31246 × 10−24 | 6.0614 × 10−16 | 26.35 | 8.27 | 6.13011 × 10−21 | 1.92395 × 10−21 | 0.68614801 |
| 2.47 × 10−10 | 8.3 × 10−16 | 0.0419906 | 5.30792 × 10−17 | 8.37273 × 10−16 | 8.73102 × 10−16 | 2.78515 × 10−15 | 1.04279179 | 2.95665 × 10−11 | 1.25096 × 10−17 | 1.082173386 | 8.37273 × 10−16 | 0.16593206 | 2.86096 × 10−24 | 8.37273 × 10−16 | 25.18 | 4.89 | 5.89137 × 10−21 | 1.14411 × 10−21 | 0.80579825 |
| 2.06 × 10−10 | 8.3 × 10−16 | 0.0436 | 5.20904 × 10−17 | 8.41025 × 10−16 | 8.69207 × 10−16 | 2.79139 × 10−15 | 1.03350884 | 2.84751 × 10−11 | 1.27471 × 10−17 | 1.085322896 | 8.41025 × 10−16 | 0.16439518 | 2.77696 × 10−24 | 8.41025 × 10−16 | 23.7 | 4.64 | 5.67654 × 10−21 | 1.11136 × 10−21 | 0.80421941 |
| 2.05 × 10−10 | 8.3 × 10−16 | 0.0452 | 5.11601 × 10−17 | 8.37123 × 10−16 | 8.73259 × 10−16 | 2.77858 × 10−15 | 1.04316772 | 2.74672 × 10−11 | 1.29789 × 10−17 | 1.088454012 | 8.37123 × 10−16 | 0.16265799 | 2.69558 × 10−24 | 8.37123 × 10−16 | 22.7 | 4.49 | 5.46567 × 10−21 | 1.08109 × 10−21 | 0.80220264 |
| 2.59 × 10−10 | 8.3 × 10−16 | 0.0468 | 5.0278 × 10−17 | 8.34624 × 10−16 | 8.75874 × 10−16 | 2.76908 × 10−15 | 1.04942362 | 2.65281 × 10−11 | 1.32066 × 10−17 | 1.091585127 | 8.34624 × 10−16 | 0.15847319 | 2.64531 × 10−24 | 8.34624 × 10−16 | 21 | 4.21 | 5.24326 × 10−21 | 1.05115 × 10−21 | 0.79952381 |
| 2.31 × 10−10 | 8.3 × 10−16 | 0.0485 | 4.93889 × 10−17 | 8.3229 × 10−16 | 8.78329 × 10−16 | 2.75992 × 10−15 | 1.05531588 | 2.55983 × 10−11 | 1.34443 × 10−17 | 1.094911937 | 8.3229 × 10−16 | 0.15663383 | 2.57166 × 10−24 | 8.3229 × 10−16 | 20.01 | 4.051 | 5.05057 × 10−21 | 1.02248 × 10−21 | 0.79755122 |
| 2.33 × 10−10 | 8.3 × 10−16 | 0.05023 | 4.8531 × 10−17 | 8.47317 × 10−16 | 8.62752 × 10−16 | 2.76591 × 10−15 | 1.0182166 | 2.47166 × 10−11 | 1.3682 × 10−17 | 1.098297456 | 8.47317 × 10−16 | 0.15420304 | 2.51204 × 10−24 | 8.47317 × 10−16 | 18.84 | 3.81 | 4.92106 × 10−21 | 9.95183 × 10−22 | 0.7977707 |
| 1.75 × 10−10 | 8.3 × 10−16 | 0.0519957 | 4.76998 × 10−17 | 8.317 × 10−16 | 8.78953 × 10−16 | 2.75151 × 10−15 | 1.05681514 | 2.38773 × 10−11 | 1.39204 × 10−17 | 1.101752838 | 8.317 × 10−16 | 0.15197547 | 2.44769 × 10−24 | 8.317 × 10−16 | 17.77 | 3.672 | 4.68879 × 10−21 | 9.68893 × 10−22 | 0.79335959 |
| 1.77 × 10−10 | 8.3 × 10−16 | 0.0537885 | 4.68982 × 10−17 | 8.31532 × 10−16 | 8.7913 × 10−16 | 2.74753 × 10−15 | 1.05724194 | 2.30814 × 10−11 | 1.41583 × 10−17 | 1.105261252 | 8.31532 × 10−16 | 0.14969171 | 2.39028 × 10−24 | 8.31532 × 10−16 | 16.76 | 3.5 | 4.52248 × 10−21 | 9.44432 × 10−22 | 0.79116945 |
| 2.47 × 10−10 | 8.3 × 10−16 | 0.0556 | 4.61278 × 10−17 | 8.26411 × 10−16 | 8.84578 × 10−16 | 2.73095 × 10−15 | 1.07038524 | 2.23294 × 10−11 | 1.43948 × 10−17 | 1.108806262 | 8.26411 × 10−16 | 0.14691267 | 2.33769 × 10−24 | 8.26411 × 10−16 | 15.9 | 3.36 | 4.35458 × 10−21 | 9.20213 × 10−22 | 0.78867925 |
| 2.26 × 10−10 | 8.3 × 10−16 | 0.0574855 | 4.53651 × 10−17 | 8.24899 × 10−16 | 8.862 × 10−16 | 2.72342 × 10−15 | 1.0743129 | 2.1597 × 10−11 | 1.46368 × 10−17 | 1.112496086 | 8.24899 × 10−16 | 0.14505794 | 2.28002 × 10−24 | 8.24899 × 10−16 | 15.14 | 3.232 | 4.20501 × 10−21 | 8.97662 × 10−22 | 0.78652576 |
| 1.7 × 10−10 | 8.3 × 10−16 | 0.0594 | 4.4628 × 10−17 | 8.12277 × 10−16 | 8.9997 × 10−16 | 2.68426 × 10−15 | 1.10796035 | 2.09009 × 10−11 | 1.48786 × 10−17 | 1.116242661 | 8.12277 × 10−16 | 0.12603407 | 2.39118 × 10−24 | 8.12277 × 10−16 | 12 | 3.12 | 4.07257 × 10−21 | 1.05887 × 10−21 | 0.74 |
| 2.22 × 10−10 | 8.3 × 10−16 | 0.0613323 | 4.39194 × 10−17 | 8.22008 × 10−16 | 8.89316 × 10−16 | 2.70845 × 10−15 | 1.08188185 | 2.02424 × 10−11 | 1.51186 × 10−17 | 1.12002407 | 8.22008 × 10−16 | 0.14092696 | 2.1777 × 10−24 | 8.22008 × 10−16 | 13.65 | 2.97 | 3.92242 × 10−21 | 8.53449 × 10−22 | 0.78241758 |
| 2.17 × 10−10 | 8.3 × 10−16 | 0.0633138 | 4.32266 × 10−17 | 8.18812 × 10−16 | 8.92787 × 10−16 | 2.69641 × 10−15 | 1.09034442 | 1.96089 × 10−11 | 1.53609 × 10−17 | 1.123901761 | 8.18812 × 10−16 | 0.13895583 | 2.12808 × 10−24 | 8.18812 × 10−16 | 13.05 | 2.874 | 3.79226 × 10−21 | 8.35169 × 10−22 | 0.77977011 |
| 2.08 × 10−10 | 8.3 × 10−16 | 0.06535 | 4.25479 × 10−17 | 7.51805 × 10−16 | 9.7236 × 10−16 | 2.7108 × 10−15 | 1.29336689 | 1.89979 × 10−11 | 1.5606 × 10−17 | 1.127886497 | 7.51805 × 10−16 | 0.13484632 | 2.11341 × 10−24 | 7.51805 × 10−16 | 12.37 | 3.55 | 3.66634 × 10−21 | 1.05218 × 10−21 | 0.71301536 |
| 2 × 10−10 | 8.3 × 10−16 | 0.0674164 | 4.18907 × 10−17 | 8.15329 × 10−16 | 8.96601 × 10−16 | 2.67938 × 10−15 | 1.09968035 | 1.84156 × 10−11 | 1.58508 × 10−17 | 1.131930333 | 8.15329 × 10−16 | 0.13484632 | 2.03786 × 10−24 | 8.15329 × 10−16 | 11.8 | 2.652 | 3.54462 × 10−21 | 7.96639 × 10−22 | 0.77525424 |
| 1.93 × 10−10 | 8.3 × 10−16 | 0.0695 | 4.1258 × 10−17 | 8.09275 × 10−16 | 9.03309 × 10−16 | 2.65959 × 10−15 | 1.11619528 | 1.78635 × 10−11 | 1.60938 × 10−17 | 1.136007828 | 8.09275 × 10−16 | 0.13189084 | 2.00212 × 10−24 | 8.09275 × 10−16 | 11.2 | 2.55 | 3.41907 × 10−21 | 7.78448 × 10−22 | 0.77232143 |
| 1.37 × 10−10 | 8.3 × 10−16 | 0.0717 | 4.06201 × 10−17 | 8.07385 × 10−16 | 9.05423 × 10−16 | 2.65044 × 10−15 | 1.12142699 | 1.73154 × 10−11 | 1.63466 × 10−17 | 1.140313112 | 8.07385 × 10−16 | 0.13013403 | 1.95789 × 10−24 | 8.07385 × 10−16 | 10.7 | 2.46 | 3.30854 × 10−21 | 7.60655 × 10−22 | 0.77009346 |
| 1.85 × 10−10 | 8.3 × 10−16 | 0.0738708 | 4.00188 × 10−17 | 8.08568 × 10−16 | 9.04099 × 10−16 | 2.64918 × 10−15 | 1.11814803 | 1.68066 × 10−11 | 1.65922 × 10−17 | 1.144561252 | 8.08568 × 10−16 | 0.12842407 | 1.91857 × 10−24 | 8.08568 × 10−16 | 10.16 | 2.36 | 3.20939 × 10−21 | 7.45488 × 10−22 | 0.76771654 |
| 1.37 × 10−10 | 8.3 × 10−16 | 0.0761 | 3.94283 × 10−17 | 8.04908 × 10−16 | 9.08209 × 10−16 | 2.63541 × 10−15 | 1.1283387 | 1.63143 × 10−11 | 1.68407 × 10−17 | 1.148923679 | 8.04908 × 10−16 | 0.1264943 | 1.87986 × 10−24 | 8.04908 × 10−16 | 9.73 | 2.28 | 3.10566 × 10−21 | 7.2774 × 10−22 | 0.76567318 |
| 1.39 × 10−10 | 8.3 × 10−16 | 0.0783948 | 3.8847 × 10−17 | 8.04572 × 10−16 | 9.08589 × 10−16 | 2.6301 × 10−15 | 1.12928159 | 1.58367 × 10−11 | 1.70927 × 10−17 | 1.153414481 | 8.04572 × 10−16 | 0.1249664 | 1.8405 × 10−24 | 8.04572 × 10−16 | 9.3 | 2.2 | 3.01269 × 10−21 | 7.12679 × 10−22 | 0.76344086 |
| 1.74 × 10−10 | 8.3 × 10−16 | 0.0807 | 3.82881 × 10−17 | 7.96314 × 10−16 | 9.18011 × 10−16 | 2.60413 × 10−15 | 1.15282559 | 1.53843 × 10−11 | 1.73422 × 10−17 | 1.157925636 | 7.96314 × 10−16 | 0.12211157 | 1.81095 × 10−24 | 7.96314 × 10−16 | 8.9 | 2.14 | 2.91143 × 10−21 | 7.00051 × 10−22 | 0.75955056 |
| 1.71 × 10−10 | 8.3 × 10−16 | 0.0831 | 3.77312 × 10−17 | 7.93123 × 10−16 | 9.21704 × 10−16 | 2.59104 × 10−15 | 1.16212053 | 1.494 × 10−11 | 1.75982 × 10−17 | 1.162622309 | 7.93123 × 10−16 | 0.11976017 | 1.78003 × 10−24 | 7.93123 × 10−16 | 8.46 | 2.06 | 2.81792 × 10−21 | 6.86161 × 10−22 | 0.75650118 |
| 1.7 × 10−10 | 8.3 × 10−16 | 0.0855 | 3.71978 × 10−17 | 7.91845 × 10−16 | 9.23192 × 10−16 | 2.58295 × 10−15 | 1.16587431 | 1.45206 × 10−11 | 1.78505 × 10−17 | 1.167318982 | 7.91845 × 10−16 | 0.11779337 | 1.74903 × 10−24 | 7.91845 × 10−16 | 8.05 | 1.98 | 2.73229 × 10−21 | 6.72041 × 10−22 | 0.75403727 |
| 1.54 × 10−10 | 8.3 × 10−16 | 0.0880045 | 3.66647 × 10−17 | 7.8682 × 10−16 | 9.29088 × 10−16 | 2.56492 × 10−15 | 1.18081349 | 1.41074 × 10−11 | 1.81101 × 10−17 | 1.172220157 | 7.8682 × 10−16 | 0.11541561 | 1.72009 × 10−24 | 7.8682 × 10−16 | 7.68 | 1.91 | 2.64241 × 10−21 | 6.57162 × 10−22 | 0.75130208 |
| 1.43 × 10−10 | 8.3 × 10−16 | 0.0905259 | 3.61505 × 10−17 | 7.81437 × 10−16 | 9.35488 × 10−16 | 2.54602 × 10−15 | 1.19713697 | 1.37145 × 10−11 | 1.83677 × 10−17 | 1.177154403 | 7.81437 × 10−16 | 0.11333337 | 1.69035 × 10−24 | 7.81437 × 10−16 | 7.38 | 1.86 | 2.56125 × 10−21 | 6.45518 × 10−22 | 0.74796748 |
| 1.35 × 10−10 | 8.3 × 10−16 | 0.0931 | 3.56472 × 10−17 | 7.78316 × 10−16 | 9.39239 × 10−16 | 2.53315 × 10−15 | 1.20675832 | 1.33353 × 10−11 | 1.8627 × 10−17 | 1.182191781 | 7.78316 × 10−16 | 0.11200213 | 1.65636 × 10−24 | 7.78316 × 10−16 | 7.14 | 1.82 | 2.48981 × 10−21 | 6.34658 × 10−22 | 0.74509804 |
| 1.27 × 10−10 | 8.3 × 10−16 | 0.0957 | 3.51597 × 10−17 | 7.67775 × 10−16 | 9.52135 × 10−16 | 2.50063 × 10−15 | 1.24012281 | 1.2973 × 10−11 | 1.88853 × 10−17 | 1.187279843 | 7.67775 × 10−16 | 0.10945739 | 1.63115 × 10−24 | 7.67775 × 10−16 | 6.9 | 1.78 | 2.40612 × 10−21 | 6.2071 × 10−22 | 0.74202899 |
| 1.2 × 10−10 | 8.3 × 10−16 | 0.0984 | 3.46739 × 10−17 | 7.82861 × 10−16 | 9.33787 × 10−16 | 2.53445 × 10−15 | 1.19278755 | 1.2617 × 10−11 | 1.91498 × 10−17 | 1.192563601 | 7.82861 × 10−16 | 0.10846882 | 1.60413 × 10−24 | 7.82861 × 10−16 | 6.37 | 1.65 | 2.34824 × 10−21 | 6.08256 × 10−22 | 0.74097331 |
| 2.7 × 10−10 | 8.3 × 10−16 | 0.101 | 3.42247 × 10−17 | 7.63351 × 10−16 | 9.57653 × 10−16 | 2.47773 × 10−15 | 1.25453865 | 1.22922 × 10−11 | 1.94012 × 10−17 | 1.197651663 | 7.63351 × 10−16 | 0.10388911 | 1.59684 × 10−24 | 7.63351 × 10−16 | 6.09 | 1.61 | 2.25513 × 10−21 | 5.96184 × 10−22 | 0.73563218 |
| 2.15 × 10−10 | 8.3 × 10−16 | 0.104 | 3.37275 × 10−17 | 7.61238 × 10−16 | 9.60311 × 10−16 | 2.46655 × 10−15 | 1.26151282 | 1.19376 × 10−11 | 1.96872 × 10−17 | 1.203522505 | 7.61238 × 10−16 | 0.10222956 | 1.56751 × 10−24 | 7.61238 × 10−16 | 5.83 | 1.56 | 2.18789 × 10−21 | 5.8544 × 10−22 | 0.73241852 |
| 1.95 × 10−10 | 8.3 × 10−16 | 0.106756 | 3.32893 × 10−17 | 7.51038 × 10−16 | 9.73353 × 10−16 | 2.43432 × 10−15 | 1.29601138 | 1.16295 × 10−11 | 1.99464 × 10−17 | 1.208915851 | 7.51038 × 10−16 | 0.09957598 | 1.54902 × 10−24 | 7.51038 × 10−16 | 5.622 | 1.53 | 2.11917 × 10−21 | 5.76722 × 10−22 | 0.72785486 |
| 1.79 × 10−10 | 8.3 × 10−16 | 0.1096 | 3.28545 × 10−17 | 7.49151 × 10−16 | 9.75804 × 10−16 | 2.42368 × 10−15 | 1.30254577 | 1.13277 × 10−11 | 2.02103 × 10−17 | 1.214481409 | 7.49151 × 10−16 | 0.09749744 | 1.52943 × 10−24 | 7.49151 × 10−16 | 5.34 | 1.47 | 2.05756 × 10−21 | 5.66408 × 10−22 | 0.7247191 |
| 1.63 × 10−10 | 8.3 × 10−16 | 0.1126 | 3.24139 × 10−17 | 7.39957 × 10−16 | 9.87929 × 10−16 | 2.39405 × 10−15 | 1.33511728 | 1.10259 × 10−11 | 2.0485 × 10−17 | 1.22035225 | 7.39957 × 10−16 | 0.09560334 | 1.50486 × 10−24 | 7.39957 × 10−16 | 5.2 | 1.45 | 1.99499 × 10−21 | 5.56294 × 10−22 | 0.72115385 |
| 1.38 × 10−10 | 8.3 × 10−16 | 0.116 | 3.19353 × 10−17 | 7.40781 × 10−16 | 9.8683 × 10−16 | 2.38955 × 10−15 | 1.33214835 | 1.07027 × 10−11 | 2.0792 × 10−17 | 1.227005871 | 7.40781 × 10−16 | 0.09357909 | 1.48255 × 10−24 | 7.40781 × 10−16 | 4.89 | 1.38 | 1.93281 × 10−21 | 5.45457 × 10−22 | 0.71779141 |
| ELEMENT | Z | N | A | E2 = E1 = E | re | ra | σ2 | σ1 | λ | (σ2 − σ1)/σ1 | b | a | rp | rn* | rn | S | pn | pe | 1 − pn − pe | pn/(pn + pe) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | MeV | m | m | cm2 | cm2 | m | % | m | m | m | m | m | ||||||||
| Na | 11 | 12 | 23 | 0.0010721 | 2.817 × 10−15 | 1.9 × 10−10 | 2.45661 × 10−19 | 2.07257 × 10−20 | 1.15802 × 10−9 | 10.8530116 | 1.29963 × 10−15 | 5.62487 × 10−16 | 7.76346 × 10−16 | 8.93475 × 10−15 | 9.196 × 10−15 | 1.24792 × 10−8 | 3.22514 × 10−10 | 2.1982 × 10−10 | 1.000 | 0.59467786 |
| Mg | 12 | 12 | 24 | 0.001305 | 2.817 × 10−15 | 1.45 × 10−10 | 2.19717 × 10−19 | 1.82828 × 10−20 | 9.51353 × 10−10 | 11.01766 | 1.39577 × 10−15 | 5.23743 × 10−16 | 8.49376 × 10−16 | 9.44581 × 10−15 | 9.722 × 10−15 | 2.1369 × 10−8 | 5.74677 × 10−10 | 3.77431 × 10−10 | 1.000 | 0.60358364 |
| Al | 13 | 14 | 27 | 0.0015596 | 2.817 × 10−15 | 1.431 × 10−10 | 1.77279 × 10−19 | 1.62226 × 10−20 | 7.96047 × 10−10 | 9.92792046 | 1.29816 × 10−15 | 5.63124 × 10−16 | 8.17954 × 10−16 | 1.04854 × 10−14 | 1.0792 × 10−14 | 2.28168 × 10−8 | 6.32574 × 10−10 | 3.8752 × 10−10 | 1.000 | 0.62011319 |
| Si | 14 | 14 | 28 | 0.00184 | 2.817 × 10−15 | 1.11 × 10−10 | 1.48796 × 10−19 | 1.44132 × 10−20 | 6.74737 × 10−10 | 9.3236246 | 1.26503 × 10−15 | 5.77871 × 10−16 | 8.07864 × 10−16 | 1.09168 × 10−14 | 1.1236 × 10−14 | 3.76522 × 10−8 | 1.07998 × 10−9 | 6.44062 × 10−10 | 1.000 | 0.62642429 |
| P | 15 | 16 | 31 | 0.00215 | 2.817 × 10−15 | 9.8 × 10−11 | 1.27025 × 10−19 | 1.28053 × 10−20 | 5.77449 × 10−10 | 8.91967871 | 1.2268 × 10−15 | 5.95879 × 10−16 | 8.09354 × 10−16 | 1.20361 × 10−14 | 1.2388 × 10−14 | 4.96501 × 10−8 | 1.47867 × 10−9 | 8.26269 × 10−10 | 1.000 | 0.64152229 |
| S | 16 | 16 | 32 | 0.00247 | 2.817 × 10−15 | 8.8 × 10−11 | 1.10214 × 10−19 | 1.15539 × 10−20 | 5.02638 × 10−10 | 8.53917051 | 1.20742 × 10−15 | 6.05442 × 10−16 | 8.05841 × 10−16 | 1.24612 × 10−14 | 1.2826 × 10−14 | 6.18233 × 10−8 | 1.87676 × 10−9 | 1.02473 × 10−9 | 1.000 | 0.64682697 |
| Cl | 17 | 18 | 35 | 0.002822 | 2.817 × 10−15 | 7.1 × 10−11 | 9.65487 × 10−20 | 1.04202 × 10−20 | 4.39942 × 10−10 | 8.26553672 | 1.17671 × 10−15 | 6.21243 × 10−16 | 8.1209 × 10−16 | 1.37783 × 10−14 | 1.4181 × 10−14 | 9.69804 × 10−8 | 3.08282 × 10−9 | 1.57419 × 10−9 | 1.000 | 0.66197398 |
| Ar | 18 | 18 | 40 | 0.0032029 | 2.817 × 10−15 | 7.9 × 10−11 | 8.45775 × 10−20 | 9.46604 × 10−21 | 3.87622 × 10−10 | 7.93482831 | 1.13459 × 10−15 | 6.44308 × 10−16 | 8.148 × 10−16 | 1.55252 × 10−14 | 1.5979 × 10−14 | 8.32144 × 10−8 | 2.69632 × 10−9 | 1.27151 × 10−9 | 1.000 | 0.6795455 |
| K | 19 | 20 | 39 | 0.0036074 | 2.817 × 10−15 | 2.43 × 10−10 | 7.79125 × 10−20 | 8.63531 × 10−21 | 3.44158 × 10−10 | 8.02255639 | 1.18406 × 10−15 | 6.17387 × 10−16 | 8.4427 × 10−16 | 1.51957 × 10−14 | 1.564 × 10−14 | 9.64851 × 10−9 | 2.80932 × 10−10 | 1.34388 × 10−10 | 1.000 | 0.67642262 |
| Ca | 20 | 20 | 40 | 0.0040381 | 2.817 × 10−15 | 1.94 × 10−10 | 6.80852 × 10−20 | 7.85343 × 10−21 | 3.0745 × 10−10 | 7.66949153 | 1.14627 × 10−15 | 6.37743 × 10−16 | 8.24093 × 10−16 | 1.55765 × 10−14 | 1.6032 × 10−14 | 1.50025 × 10−8 | 4.48103 × 10−10 | 2.10848 × 10−10 | 1.000 | 0.68002422 |
| Sc | 21 | 24 | 45 | 0.00449 | 2.817 × 10−15 | 1.84 × 10−10 | 6.08462 × 10−20 | 7.23435 × 10−21 | 2.76507 × 10−10 | 7.41073271 | 1.1077 × 10−15 | 6.5995 × 10−16 | 8.27451 × 10−16 | 1.74731 × 10−14 | 1.7984 × 10−14 | 1.74478 × 10−8 | 5.37788 × 10−10 | 2.34389 × 10−10 | 1.000 | 0.69645647 |
| Ti | 22 | 26 | 48 | 0.00497 | 2.817 × 10−15 | 1.76 × 10−10 | 5.47006 × 10−20 | 6.66266 × 10−21 | 2.49802 × 10−10 | 7.21002387 | 1.08494 × 10−15 | 6.73794 × 10−16 | 8.27627 × 10−16 | 1.86079 × 10−14 | 1.9152 × 10−14 | 1.95297 × 10−8 | 6.12971 × 10−10 | 2.56182 × 10−10 | 1.000 | 0.70525109 |
| V | 23 | 28 | 51 | 0.0054651 | 2.817 × 10−15 | 1.71 × 10−10 | 4.96531 × 10−20 | 6.15546 × 10−21 | 2.27172 × 10−10 | 7.06651092 | 1.06738 × 10−15 | 6.8488 × 10−16 | 8.31218 × 10−16 | 1.97971 × 10−14 | 2.0376 × 10−14 | 2.12141 × 10−8 | 6.76721 × 10−10 | 2.71382 × 10−10 | 1.000 | 0.71376292 |
| Cr | 24 | 28 | 52 | 0.0059892 | 2.817 × 10−15 | 1.66 × 10−10 | 5.16003 × 10−20 | 5.67543 × 10−21 | 2.07292 × 10−10 | 8.09187709 | 1.23024 × 10−15 | 5.94215 × 10−16 | 9.64581 × 10−16 | 2.02052 × 10−14 | 2.0796 × 10−14 | 2.26586 × 10−8 | 7.27935 × 10−10 | 2.87977 × 10−10 | 1.000 | 0.71653377 |
| Mn | 25 | 30 | 55 | 0.00654 | 2.817 × 10−15 | 1.61 × 10−10 | 4.1236 × 10−20 | 5.29134 × 10−21 | 1.89834 × 10−10 | 6.79310345 | 1.04067 × 10−15 | 7.02456 × 10−16 | 8.31098 × 10−16 | 2.13517 × 10−14 | 2.1976 × 10−14 | 2.46297 × 10−8 | 8.02854 × 10−10 | 3.06141 × 10−10 | 1.000 | 0.72394712 |
| Fe | 26 | 30 | 56 | 0.00711 | 2.817 × 10−15 | 1.558 × 10−10 | 3.78384 × 10−20 | 4.93383 × 10−21 | 1.74615 × 10−10 | 6.66917293 | 1.03257 × 10−15 | 7.07967 × 10−16 | 8.29156 × 10−16 | 2.17053 × 10−14 | 2.234 × 10−14 | 2.64205 × 10−8 | 8.66782 × 10−10 | 3.26918 × 10−10 | 1.000 | 0.72613046 |
| Co | 27 | 32 | 59 | 0.00771 | 2.817 × 10−15 | 1.52 × 10−10 | 3.48366 × 10−20 | 4.609 × 10−21 | 1.61027 × 10−10 | 6.55838641 | 1.01871 × 10−15 | 7.176 × 10−16 | 8.32793 × 10−16 | 2.29023 × 10−14 | 2.3572 × 10−14 | 2.83862 × 10−8 | 9.43843 × 10−10 | 3.43468 × 10−10 | 1.000 | 0.7331894 |
| Ni | 28 | 30 | 59 | 0.00833 | 2.817 × 10−15 | 1.49 × 10−10 | 3.20634 × 10−20 | 4.31735 × 10−21 | 1.49041 × 10−10 | 6.42663657 | 1.01101 × 10−15 | 7.23068 × 10−16 | 8.25372 × 10−16 | 2.28091 × 10−14 | 2.3476 × 10−14 | 2.9432 × 10−8 | 9.79564 × 10−10 | 3.57438 × 10−10 | 1.000 | 0.73265684 |
| Cu | 29 | 34 | 64 | 0.0089789 | 2.817 × 10−15 | 1.45 × 10−10 | 2.93366 × 10−20 | 4.0417 × 10−21 | 1.3827 × 10−10 | 6.25848564 | 9.7977 × 10−16 | 7.46119 × 10−16 | 8.21366 × 10−16 | 2.46978 × 10−14 | 2.542 × 10−14 | 3.21656 × 10−8 | 1.0907 × 10−9 | 3.77431 × 10−10 | 1.000 | 0.74291651 |
| Zn | 30 | 34 | 65 | 0.00966 | 2.817 × 10−15 | 1.42 × 10−10 | 2.7588 × 10−20 | 3.81236 × 10−21 | 1.28521 × 10−10 | 6.23646724 | 9.85932 × 10−16 | 7.41456 × 10−16 | 8.34513 × 10−16 | 2.54203 × 10−14 | 2.6164 × 10−14 | 3.39294 × 10−8 | 1.15934 × 10−9 | 3.93547 × 10−10 | 1.000 | 0.74657056 |
| Ga | 31 | 38 | 70 | 0.0103671 | 2.817 × 10−15 | 1.35 × 10−10 | 2.55858 × 10−20 | 3.58896 × 10−21 | 1.19755 × 10−10 | 6.12903226 | 9.68602 × 10−16 | 7.54722 × 10−16 | 8.37472 × 10−16 | 2.70957 × 10−14 | 2.7888 × 10−14 | 3.85059 × 10−8 | 1.33844 × 10−9 | 4.35418 × 10−10 | 1.000 | 0.75453666 |
| Ge | 32 | 41 | 73 | 0.0111 | 2.817 × 10−15 | 1.52 × 10−10 | 2.38831 × 10−20 | 3.38947 × 10−21 | 1.11848 × 10−10 | 6.04626335 | 9.59806 × 10−16 | 7.61639 × 10−16 | 8.41293 × 10−16 | 2.82305 × 10−14 | 2.9056 × 10−14 | 3.13111 × 10−8 | 1.08507 × 10−9 | 3.43468 × 10−10 | 1.000 | 0.75956728 |
| As | 33 | 42 | 75 | 0.0118667 | 2.817 × 10−15 | 1.14 × 10−10 | 2.22939 × 10−20 | 3.20599 × 10−21 | 1.04622 × 10−10 | 5.95382227 | 9.51031 × 10−16 | 7.68666 × 10−16 | 8.42233 × 10−16 | 2.91166 × 10−14 | 2.9968 × 10−14 | 5.50083 × 10−8 | 1.96918 × 10−9 | 6.1061 × 10−10 | 1.000 | 0.76330983 |
| Se | 34 | 44 | 79 | 0.0126578 | 2.817 × 10−15 | 1.03 × 10−10 | 2.08344 × 10−20 | 3.03927 × 10−21 | 9.8083 × 10−11 | 5.85504745 | 9.38025 × 10−16 | 7.79324 × 10−16 | 8.45385 × 10−16 | 3.06867 × 10−14 | 3.1584 × 10−14 | 6.84478 × 10−8 | 2.49819 × 10−9 | 7.47996 × 10−10 | 1.000 | 0.76957726 |
| Br | 35 | 44 | 80 | 0.0134737 | 2.817 × 10−15 | 1.35 × 10−10 | 1.95168 × 10−20 | 2.88706 × 10−21 | 9.21436 × 10−11 | 5.76011029 | 9.31411 × 10−16 | 7.84858 × 10−16 | 8.42742 × 10−16 | 3.1052 × 10−14 | 3.196 × 10−14 | 4.11011 × 10−8 | 1.46575 × 10−9 | 4.35418 × 10−10 | 1.000 | 0.77097334 |
| Kr | 36 | 48 | 84 | 0.0143256 | 2.817 × 10−15 | 1.9 × 10−10 | 1.82708 × 10−20 | 2.74271 × 10−21 | 8.66641 × 10−11 | 5.6615931 | 9.18076 × 10−16 | 7.96258 × 10−16 | 8.43976 × 10−16 | 3.25677 × 10−14 | 3.352 × 10−14 | 2.19077 × 10−8 | 7.63867 × 10−10 | 2.1982 × 10−10 | 1.000 | 0.77653498 |
| Rb | 37 | 48 | 85 | 0.015199 | 2.817 × 10−15 | 2.65 × 10−10 | 1.71731 × 10−20 | 2.61145 × 10−21 | 8.1684 × 10−11 | 5.57608696 | 9.10756 × 10−16 | 8.02657 × 10−16 | 8.42772 × 10−16 | 3.32168 × 10−14 | 3.4188 × 10−14 | 1.1711 × 10−8 | 3.97875 × 10−10 | 1.13001 × 10−10 | 1.000 | 0.77880943 |
| Sr | 38 | 50 | 88 | 0.0161046 | 2.817 × 10−15 | 2.19 × 10−10 | 1.6121 × 10−20 | 2.49381 × 10−21 | 7.70907 × 10−11 | 5.46441074 | 8.99814 × 10−16 | 8.12418 × 10−16 | 8.39569 × 10−16 | 3.40523 × 10−14 | 3.5048 × 10−14 | 1.70689 × 10−8 | 5.92302 × 10−10 | 1.65457 × 10−10 | 1.000 | 0.78164941 |
| Y | 39 | 50 | 89 | 0.0170384 | 2.817 × 10−15 | 2.12 × 10−10 | 1.5192 × 10−20 | 2.37993 × 10−21 | 7.28657 × 10−11 | 5.38337469 | 8.93149 × 10−16 | 8.1848 × 10−16 | 8.3742 × 10−16 | 3.45537 × 10−14 | 3.5564 × 10−14 | 1.82932 × 10−8 | 6.38251 × 10−10 | 1.76564 × 10−10 | 1.000 | 0.78330781 |
| Zr | 40 | 51 | 91 | 0.017997 | 2.817 × 10−15 | 2.06 × 10−10 | 1.43446 × 10−20 | 2.27212 × 10−21 | 6.89846 × 10−11 | 5.31333333 | 8.85676 × 10−16 | 8.25386 × 10−16 | 8.37543 × 10−16 | 3.54514 × 10−14 | 3.6488 × 10−14 | 1.95708 × 10−8 | 6.8763 × 10−10 | 1.86999 × 10−10 | 1.000 | 0.7861962 |
| Nb | 41 | 52 | 93 | 0.0189856 | 2.817 × 10−15 | 1.98 × 10−10 | 1.3552 × 10−20 | 2.17382 × 10−21 | 6.53925 × 10−11 | 5.23420866 | 8.78303 × 10−16 | 8.32315 × 10−16 | 8.35668 × 10−16 | 3.61082 × 10−14 | 3.7164 × 10−14 | 2.13019 × 10−8 | 7.53484 × 10−10 | 2.02415 × 10−10 | 1.000 | 0.78824611 |
| Mo | 42 | 54 | 96 | 0.019999 | 2.817 × 10−15 | 1.9 × 10−10 | 1.28406 × 10−20 | 2.08699 × 10−21 | 6.20789 × 10−11 | 5.15267176 | 8.70475 × 10−16 | 8.398 × 10−16 | 8.37126 × 10−16 | 3.72858 × 10−14 | 3.8376 × 10−14 | 2.34179 × 10−8 | 8.35966 × 10−10 | 2.1982 × 10−10 | 1.000 | 0.79179513 |
| Tc | 43 | 55 | 99 | 0.021044 | 2.817 × 10−15 | 1.83 × 10−10 | 1.22866 × 10−20 | 2.01848 × 10−21 | 5.89962 × 10−11 | 5.08706265 | 8.70953 × 10−16 | 8.39339 × 10−16 | 8.4613 × 10−16 | 3.84386 × 10−14 | 3.9563 × 10−14 | 2.5543 × 10−8 | 9.19623 × 10−10 | 2.36958 × 10−10 | 1.000 | 0.79512187 |
| Ru | 44 | 57 | 101 | 0.0221 | 2.817 × 10−15 | 2.65 × 10−10 | 1.15468 × 10−20 | 1.91327 × 10−21 | 5.61772 × 10−11 | 5.03508772 | 8.55606 × 10−16 | 8.54395 × 10−16 | 8.37237 × 10−16 | 3.92795 × 10−14 | 4.0428 × 10−14 | 1.27267 × 10−8 | 4.44924 × 10−10 | 1.13001 × 10−10 | 1.000 | 0.79746206 |
| Rh | 45 | 58 | 103 | 0.0232 | 2.817 × 10−15 | 1.34 × 10−10 | 1.09538 × 10−20 | 1.84557 × 10−21 | 5.35136 × 10−11 | 4.93518519 | 8.47163 × 10−16 | 8.62909 × 10−16 | 8.33976 × 10−16 | 3.99946 × 10−14 | 4.1164 × 10−14 | 4.7222 × 10−8 | 1.76113 × 10−9 | 4.41941 × 10−10 | 1.000 | 0.79939777 |
| Pd | 46 | 60 | 108 | 0.0244 | 2.817 × 10−15 | 1.69 × 10−10 | 1.05681 × 10−20 | 1.7912 × 10−21 | 5.08818 × 10−11 | 4.9 | 8.53248 × 10−16 | 8.56756 × 10−16 | 8.53243 × 10−16 | 4.19215 × 10−14 | 4.3147 × 10−14 | 3.10579 × 10−8 | 1.14249 × 10−9 | 2.77844 × 10−10 | 1.000 | 0.80438121 |
| Ag | 47 | 60 | 108 | 0.025514 | 2.817 × 10−15 | 1.65 × 10−10 | 9.92435 × 10−21 | 1.71826 × 10−21 | 4.86602 × 10−11 | 4.77580813 | 8.3415 × 10−16 | 8.76371 × 10−16 | 8.34228 × 10−16 | 4.19339 × 10−14 | 4.316 × 10−14 | 3.25154 × 10−8 | 1.19879 × 10−9 | 2.91478 × 10−10 | 1.000 | 0.80441203 |
| Cd | 48 | 64 | 112 | 0.0267 | 2.817 × 10−15 | 1.61 × 10−10 | 9.45448 × 10−21 | 1.64431 × 10−21 | 4.64987 × 10−11 | 4.74980134 | 8.26432 × 10−16 | 8.84556 × 10−16 | 8.37868 × 10−16 | 4.3687 × 10−14 | 4.4964 × 10−14 | 3.47881 × 10−8 | 1.29395 × 10−9 | 3.06141 × 10−10 | 1.000 | 0.80867228 |
| In | 49 | 64 | 115 | 0.0279 | 2.817 × 10−15 | 1.56 × 10−10 | 9.0168 × 10−21 | 1.58604 × 10−21 | 4.44988 × 10−11 | 4.68509615 | 8.19435 × 10−16 | 8.92109 × 10−16 | 8.36618 × 10−16 | 4.46155 × 10−14 | 4.592 × 10−14 | 3.73432 × 10−8 | 1.39768 × 10−9 | 3.2608 × 10−10 | 1.000 | 0.81083206 |
| Sn | 50 | 69 | 119 | 0.0292001 | 2.817 × 10−15 | 1.45 × 10−10 | 8.59455 × 10−21 | 1.52967 × 10−21 | 4.25175 × 10−11 | 4.6185567 | 8.11986 × 10−16 | 9.00293 × 10−16 | 8.38319 × 10−16 | 4.6135 × 10−14 | 4.7484 × 10−14 | 4.36711 × 10−8 | 1.65431 × 10−9 | 3.77431 × 10−10 | 1.000 | 0.81423307 |
| Sb | 51 | 70 | 122 | 0.030491 | 2.817 × 10−15 | 1.33 × 10−10 | 8.2378 × 10−21 | 1.47848 × 10−21 | 4.07174 × 10−11 | 4.57181943 | 8.08652 × 10−16 | 9.04005 × 10−16 | 8.42058 × 10−16 | 4.73359 × 10−14 | 4.872 × 10−14 | 5.21708 × 10−8 | 2.00028 × 10−9 | 4.48612 × 10−10 | 1.000 | 0.81681036 |
| Te | 52 | 76 | 128 | 0.0318 | 2.817 × 10−15 | 1.23 × 10−10 | 7.88212 × 10−21 | 1.4281 × 10−21 | 3.90414 × 10−11 | 4.51928783 | 8.00769 × 10−16 | 9.12903 × 10−16 | 8.4688 × 10−16 | 4.959 × 10−14 | 5.104 × 10−14 | 6.2014 × 10−8 | 2.41242 × 10−9 | 5.24522 × 10−10 | 1.000 | 0.82140541 |
| I | 53 | 74 | 127 | 0.0331694 | 2.817 × 10−15 | 1.15 × 10−10 | 7.54808 × 10−21 | 1.38023 × 10−21 | 3.74295 × 10−11 | 4.46870229 | 7.98742 × 10−16 | 9.1522 × 10−16 | 8.43188 × 10−16 | 4.9318 × 10−14 | 5.076 × 10−14 | 7.02911 × 10−8 | 2.74963 × 10−9 | 6.00037 × 10−10 | 1.000 | 0.82086678 |
| Xe | 54 | 77 | 131 | 0.0356 | 2.817 × 10−15 | 1.08 × 10−10 | 7.23856 × 10−21 | 1.33652 × 10−21 | 3.4874 × 10−11 | 4.41598695 | 8.17027 × 10−16 | 8.94738 × 10−16 | 8.72345 × 10−16 | 5.1028 × 10−14 | 5.252 × 10−14 | 8.06237 × 10−8 | 3.18927 × 10−9 | 6.8034 × 10−10 | 1.000 | 0.82418383 |
| Cs | 55 | 78 | 133 | 0.0359 | 2.817 × 10−15 | 2.98 × 10−10 | 6.92953 × 10−21 | 1.29322 × 10−21 | 3.45826 × 10−11 | 4.35836177 | 7.85704 × 10−16 | 9.30407 × 10−16 | 8.42295 × 10−16 | 5.16498 × 10−14 | 5.316 × 10−14 | 1.16946 × 10−8 | 4.22292 × 10−10 | 8.93596 × 10−11 | 1.000 | 0.82535085 |
| Ba | 56 | 81 | 137 | 0.0374 | 2.817 × 10−15 | 2.53 × 10−10 | 6.65737 × 10−21 | 1.25396 × 10−21 | 3.31956 × 10−11 | 4.30909091 | 7.81766 × 10−16 | 9.35094 × 10−16 | 8.47221 × 10−16 | 5.33598 × 10−14 | 5.492 × 10−14 | 1.62656 × 10−8 | 5.98736 × 10−10 | 1.23975 × 10−10 | 1.000 | 0.82845881 |
| La | 57 | 82 | 139 | 0.0389 | 2.817 × 10−15 | 1.95 × 10−10 | 6.38898 × 10−21 | 1.21552 × 10−21 | 3.19156 × 10−11 | 4.25616698 | 7.77489 × 10−16 | 9.40239 × 10−16 | 8.45845 × 10−16 | 5.39816 × 10−14 | 5.556 × 10−14 | 2.69088 × 10−8 | 1.01569 × 10−9 | 2.08691 × 10−10 | 1.000 | 0.82955371 |
| Ce | 58 | 82 | 140 | 0.03598 | 2.817 × 10−15 | 2.98 × 10−10 | 6.13011 × 10−21 | 1.92395 × 10−21 | 3.45057 × 10−11 | 2.18621524 | 5.841 × 10−16 | 1.25154 × 10−15 | 6.37278 × 10−16 | 5.4448 × 10−14 | 5.604 × 10−14 | 1.20207 × 10−8 | 4.3741 × 10−10 | 8.93596 × 10−11 | 1.000 | 0.83036305 |
| Pr | 59 | 82 | 141 | 0.0419906 | 2.817 × 10−15 | 2.47 × 10−10 | 5.89137 × 10−21 | 1.14411 × 10−21 | 2.95665 × 10−11 | 4.14928425 | 7.68874 × 10−16 | 9.50773 × 10−16 | 8.40469 × 10−16 | 5.47589 × 10−14 | 5.636 × 10−14 | 1.72605 × 10−8 | 6.3911 × 10−10 | 1.30071 × 10−10 | 1.000 | 0.83089708 |
| Nd | 60 | 83 | 144 | 0.0436 | 2.817 × 10−15 | 2.06 × 10−10 | 5.67654 × 10−21 | 1.11136 × 10−21 | 2.84751 × 10−11 | 4.10775862 | 7.65712 × 10−16 | 9.54699 × 10−16 | 8.43574 × 10−16 | 5.60569 × 10−14 | 5.7696 × 10−14 | 2.47144 × 10−8 | 9.33294 × 10−10 | 1.86999 × 10−10 | 1.000 | 0.8330803 |
| Pm | 61 | 84 | 145 | 0.0452 | 2.817 × 10−15 | 2.05 × 10−10 | 5.46567 × 10−21 | 1.08109 × 10−21 | 2.74672 × 10−11 | 4.05567929 | 7.61962 × 10−16 | 9.59398 × 10−16 | 8.40914 × 10−16 | 5.63523 × 10−14 | 5.8 × 10−14 | 2.50138 × 10−8 | 9.45729 × 10−10 | 1.88828 × 10−10 | 1.000 | 0.83356691 |
| Sm | 62 | 88 | 150 | 0.0468 | 2.817 × 10−15 | 2.59 × 10−10 | 5.24326 × 10−21 | 1.05115 × 10−21 | 2.65281 × 10−11 | 3.98812352 | 7.51301 × 10−16 | 9.73012 × 10−16 | 8.39241 × 10−16 | 5.84354 × 10−14 | 6.0144 × 10−14 | 1.63112 × 10−8 | 6.06994 × 10−10 | 1.18297 × 10−10 | 1.000 | 0.83689709 |
| Eu | 63 | 88 | 152 | 0.0485 | 2.817 × 10−15 | 2.31 × 10−10 | 5.05057 × 10−21 | 1.02248 × 10−21 | 2.55983 × 10−11 | 3.93952111 | 7.47249 × 10−16 | 9.78288 × 10−16 | 8.37738 × 10−16 | 5.90727 × 10−14 | 6.08 × 10−14 | 2.04128 × 10−8 | 7.68601 × 10−10 | 1.48713 × 10−10 | 1.000 | 0.83788186 |
| Gd | 64 | 93 | 157 | 0.05023 | 2.817 × 10−15 | 2.33 × 10−10 | 4.92106 × 10−21 | 9.95183 × 10−22 | 2.47166 × 10−11 | 3.94488189 | 7.51505 × 10−16 | 9.72748 × 10−16 | 8.52189 × 10−16 | 6.11325 × 10−14 | 6.292 × 10−14 | 2.04459 × 10−8 | 7.72923 × 10−10 | 1.46171 × 10−10 | 1.000 | 0.84096174 |
| Tb | 65 | 94 | 159 | 0.0519957 | 2.817 × 10−15 | 1.75 × 10−10 | 4.68879 × 10−21 | 9.68893 × 10−22 | 2.38773 × 10−11 | 3.83932462 | 7.36318 × 10−16 | 9.92812 × 10−16 | 8.37788 × 10−16 | 6.17543 × 10−14 | 6.356 × 10−14 | 3.5501 × 10−8 | 1.37944 × 10−9 | 2.59118 × 10−10 | 1.000 | 0.84186202 |
| Dy | 66 | 97 | 163 | 0.0537885 | 2.817 × 10−15 | 1.77 × 10−10 | 4.52248 × 10−21 | 9.44432 × 10−22 | 2.30814 × 10−11 | 3.78857143 | 7.30943 × 10−16 | 1.00011 × 10−15 | 8.37906 × 10−16 | 6.31534 × 10−14 | 6.5 × 10−14 | 3.51539 × 10−8 | 1.36873 × 10−9 | 2.53295 × 10−10 | 1.000 | 0.84384022 |
| Ho | 67 | 98 | 165 | 0.0556 | 2.817 × 10−15 | 2.47 × 10−10 | 4.35458 × 10−21 | 9.20213 × 10−22 | 2.23294 × 10−11 | 3.73214286 | 7.24104 × 10−16 | 1.00956 × 10−15 | 8.34182 × 10−16 | 6.40978 × 10−14 | 6.5972 × 10−14 | 1.87545 × 10−8 | 7.09853 × 10−10 | 1.30071 × 10−10 | 1.000 | 0.84513975 |
| Er | 68 | 99 | 167 | 0.0574855 | 2.817 × 10−15 | 2.26 × 10−10 | 4.20501 × 10−21 | 8.97662 × 10−22 | 2.1597 × 10−11 | 3.68440594 | 7.19932 × 10−16 | 1.01541 × 10−15 | 8.33264 × 10−16 | 6.50033 × 10−14 | 6.6904 × 10−14 | 2.23898 × 10−8 | 8.55868 × 10−10 | 1.55366 × 10−10 | 1.000 | 0.84635981 |
| Tm | 69 | 122 | 204 | 0.0594 | 2.817 × 10−15 | 1.7 × 10−10 | 4.07257 × 10−21 | 1.05887 × 10−21 | 2.09009 × 10−11 | 2.84615385 | 6.64834 × 10−16 | 1.09956 × 10−15 | 8.22652 × 10−16 | 7.94295 × 10−14 | 8.1752 × 10−14 | 4.29295 × 10−8 | 1.72885 × 10−9 | 2.74584 × 10−10 | 1.000 | 0.86294305 |
| Yb | 70 | 103 | 173 | 0.0613323 | 2.817 × 10−15 | 2.22 × 10−10 | 3.92242 × 10−21 | 8.53449 × 10−22 | 2.02424 × 10−11 | 3.5959596 | 7.10289 × 10−16 | 1.02919 × 10−15 | 8.3148 × 10−16 | 6.72535 × 10−14 | 6.922 × 10−14 | 2.35905 × 10−8 | 9.07342 × 10−10 | 1.61016 × 10−10 | 1.000 | 0.84928683 |
| Lu | 71 | 104 | 175 | 0.0633138 | 2.817 × 10−15 | 2.17 × 10−10 | 3.79226 × 10−21 | 8.35169 × 10−22 | 1.96089 × 10−11 | 3.54070981 | 7.05715 × 10−16 | 1.03586 × 10−15 | 8.29216 × 10−16 | 6.80114 × 10−14 | 7 × 10−14 | 2.47871 × 10−8 | 9.56757 × 10−10 | 1.68521 × 10−10 | 1.000 | 0.85024053 |
| Hf | 72 | 72 | 178 | 0.06535 | 2.817 × 10−15 | 2.08 × 10−10 | 3.66634 × 10−21 | 1.05218 × 10−21 | 1.89979 × 10−11 | 2.48450704 | 6.42676 × 10−16 | 1.13747 × 10−15 | 7.60133 × 10−16 | 6.93677 × 10−14 | 7.1396 × 10−14 | 2.71601 × 10−8 | 1.05514 × 10−9 | 1.8342 × 10−10 | 1.000 | 0.85190905 |
| Ta | 73 | 108 | 181 | 0.0674164 | 2.817 × 10−15 | 2 × 10−10 | 3.54462 × 10−21 | 7.96639 × 10−22 | 1.84156 × 10−11 | 3.4494721 | 6.9602 × 10−16 | 1.05029 × 10−15 | 8.26913 × 10−16 | 7.03043 × 10−14 | 7.236 × 10−14 | 2.94823 × 10−8 | 1.15149 × 10−9 | 1.98387 × 10−10 | 1.000 | 0.85303353 |
| W | 74 | 109 | 184 | 0.0695 | 2.817 × 10−15 | 1.93 × 10−10 | 3.41907 × 10−21 | 7.78448 × 10−22 | 1.78635 × 10−11 | 3.39215686 | 6.88413 × 10−16 | 1.0619 × 10−15 | 8.22283 × 10−16 | 7.14469 × 10−14 | 7.3536 × 10−14 | 3.18304 × 10−8 | 1.2499 × 10−9 | 2.13039 × 10−10 | 1.000 | 0.85437582 |
| Re | 75 | 110 | 186 | 0.0717 | 2.817 × 10−15 | 1.37 × 10−10 | 3.30854 × 10−21 | 7.60655 × 10−22 | 1.73154 × 10−11 | 3.3495935 | 6.84415 × 10−16 | 1.0681 × 10−15 | 8.21005 × 10−16 | 7.2368 × 10−14 | 7.4484 × 10−14 | 6.16021 × 10−8 | 2.50182 × 10−9 | 4.22798 × 10−10 | 1.000 | 0.85543508 |
| Os | 76 | 114 | 190 | 0.0738708 | 2.817 × 10−15 | 1.85 × 10−10 | 3.20939 × 10−21 | 7.45488 × 10−22 | 1.68066 × 10−11 | 3.30508475 | 6.805 × 10−16 | 1.07425 × 10−15 | 8.22141 × 10−16 | 7.39303 × 10−14 | 7.6092 × 10−14 | 3.51475 × 10−8 | 1.39168 × 10−9 | 2.31862 × 10−10 | 1.000 | 0.85718709 |
| Ir | 77 | 114 | 192 | 0.0761 | 2.817 × 10−15 | 1.37 × 10−10 | 3.10566 × 10−21 | 7.2774 × 10−22 | 1.63143 × 10−11 | 3.26754386 | 6.75906 × 10−16 | 1.08155 × 10−15 | 8.19423 × 10−16 | 7.47025 × 10−14 | 7.6887 × 10−14 | 6.26615 × 10−8 | 2.55534 × 10−9 | 4.22798 × 10−10 | 1.000 | 0.85803302 |
| Pt | 78 | 117 | 195 | 0.0783948 | 2.817 × 10−15 | 1.39 × 10−10 | 3.01269 × 10−21 | 7.12679 × 10−22 | 1.58367 × 10−11 | 3.22727273 | 6.72535 × 10−16 | 1.08697 × 10−15 | 8.19369 × 10−16 | 7.58167 × 10−14 | 7.8034 × 10−14 | 6.14429 × 10−8 | 2.50696 × 10−9 | 4.10718 × 10−10 | 1.000 | 0.85923109 |
| Au | 79 | 118 | 197 | 0.0807 | 2.817 × 10−15 | 1.74 × 10−10 | 2.91143 × 10−21 | 7.00051 × 10−22 | 1.53843 × 10−11 | 3.1588785 | 6.65023 × 10−16 | 1.09925 × 10−15 | 8.12861 × 10−16 | 7.65614 × 10−14 | 7.88 × 10−14 | 4.02586 × 10−8 | 1.61031 × 10−9 | 2.62105 × 10−10 | 1.000 | 0.86001734 |
| Hg | 80 | 121 | 201 | 0.0831 | 2.817 × 10−15 | 1.71 × 10−10 | 2.81792 × 10−21 | 6.86161 × 10−22 | 1.494 × 10−11 | 3.10679612 | 6.59041 × 10−16 | 1.10923 × 10−15 | 8.10413 × 10−16 | 7.79566 × 10−14 | 8.0236 × 10−14 | 4.20242 × 10−8 | 1.6875 × 10−9 | 2.71382 × 10−10 | 1.000 | 0.86146047 |
| Tl | 81 | 122 | 204 | 0.0855 | 2.817 × 10−15 | 1.7 × 10−10 | 2.73229 × 10−21 | 6.72041 × 10−22 | 1.45206 × 10−11 | 3.06565657 | 6.54195 × 10−16 | 1.11744 × 10−15 | 8.09514 × 10−16 | 7.94373 × 10−14 | 8.176 × 10−14 | 4.29318 × 10−8 | 1.72896 × 10−9 | 2.74584 × 10−10 | 1.000 | 0.86295076 |
| Pb | 82 | 125 | 207 | 0.0880045 | 2.817 × 10−15 | 1.54 × 10−10 | 2.64241 × 10−21 | 6.57162 × 10−22 | 1.41074 × 10−11 | 3.02094241 | 6.48043 × 10−16 | 1.12805 × 10−15 | 8.05547 × 10−16 | 8.05255 × 10−14 | 8.288 × 10−14 | 5.22169 × 10−8 | 2.12609 × 10−9 | 3.34605 × 10−10 | 1.000 | 0.86401997 |
| Bi | 83 | 126 | 209 | 0.0905259 | 2.817 × 10−15 | 1.43 × 10−10 | 2.56125 × 10−21 | 6.45518 × 10−22 | 1.37145 × 10−11 | 2.96774194 | 6.42765 × 10−16 | 1.13731 × 10−15 | 8.01293 × 10−16 | 8.1225 × 10−14 | 8.36 × 10−14 | 6.04189 × 10−8 | 2.48002 × 10−9 | 3.88062 × 10−10 | 1.000 | 0.86469605 |
| Po | 84 | 125 | 210 | 0.0931 | 2.817 × 10−15 | 1.35 × 10−10 | 2.48981 × 10−21 | 6.34658 × 10−22 | 1.33353 × 10−11 | 2.92307692 | 6.39864 × 10−16 | 1.14247 × 10−15 | 7.98946 × 10−16 | 8.16136 × 10−14 | 8.4 × 10−14 | 6.7596 × 10−8 | 2.79152 × 10−9 | 4.35418 × 10−10 | 1.000 | 0.86506792 |
| At | 85 | 125 | 210 | 0.0957 | 2.817 × 10−15 | 1.27 × 10−10 | 2.40612 × 10−21 | 6.2071 × 10−22 | 1.2973 × 10−11 | 2.87640449 | 6.33007 × 10−16 | 1.15485 × 10−15 | 7.90384 × 10−16 | 8.16136 × 10−14 | 8.4 × 10−14 | 7.59347 × 10−8 | 3.15429 × 10−9 | 4.92001 × 10−10 | 1.000 | 0.86506792 |
| Rn | 86 | 136 | 222 | 0.0984 | 2.817 × 10−15 | 1.2 × 10−10 | 2.34824 × 10−21 | 6.08256 × 10−22 | 1.2617 × 10−11 | 2.86060606 | 6.31191 × 10−16 | 1.15817 × 10−15 | 8.0285 × 10−16 | 8.62773 × 10−14 | 8.88 × 10−14 | 8.71816 × 10−8 | 3.66636 × 10−9 | 5.51076 × 10−10 | 1.000 | 0.86933401 |
| Fr | 87 | 136 | 223 | 0.101 | 2.817 × 10−15 | 2.7 × 10−10 | 2.25513 × 10−21 | 5.96184 × 10−22 | 1.22922 × 10−11 | 2.7826087 | 6.17455 × 10−16 | 1.18393 × 10−15 | 7.86555 × 10−16 | 8.66659 × 10−14 | 8.92 × 10−14 | 1.86179 × 10−8 | 7.26393 × 10−10 | 1.08854 × 10−10 | 1.000 | 0.86967399 |
| Ra | 88 | 138 | 226 | 0.104 | 2.817 × 10−15 | 2.15 × 10−10 | 2.18789 × 10−21 | 5.8544 × 10−22 | 1.19376 × 10−11 | 2.73717949 | 6.13435 × 10−16 | 1.19169 × 10−15 | 7.84923 × 10−16 | 8.78318 × 10−14 | 9.04 × 10−14 | 2.89726 × 10−8 | 1.15582 × 10−9 | 1.71671 × 10−10 | 1.000 | 0.8706804 |
| Ac | 89 | 138 | 227 | 0.106756 | 2.817 × 10−15 | 1.95 × 10−10 | 2.11917 × 10−21 | 5.76722 × 10−22 | 1.16295 × 10−11 | 2.6745098 | 6.05881 × 10−16 | 1.20655 × 10−15 | 7.76399 × 10−16 | 8.82205 × 10−14 | 9.08 × 10−14 | 3.49898 × 10−8 | 1.40921 × 10−9 | 2.08691 × 10−10 | 1.000 | 0.87101145 |
| Th | 90 | 142 | 232 | 0.1096 | 2.817 × 10−15 | 1.79 × 10−10 | 2.05756 × 10−21 | 5.66408 × 10−22 | 1.13277 × 10−11 | 2.63265306 | 6.0021 × 10−16 | 1.21795 × 10−15 | 7.74782 × 10−16 | 9.01792 × 10−14 | 9.2816 × 10−14 | 4.1694 × 10−8 | 1.69706 × 10−9 | 2.47667 × 10−10 | 1.000 | 0.87264733 |
| Pa | 91 | 140 | 231 | 0.1126 | 2.817 × 10−15 | 1.63 × 10−10 | 1.99499 × 10−21 | 5.56294 × 10−22 | 1.10259 × 10−11 | 2.5862069 | 5.95162 × 10−16 | 1.22828 × 10−15 | 7.6716 × 10−16 | 8.97905 × 10−14 | 9.2416 × 10−14 | 4.97241 × 10−8 | 2.0407 × 10−9 | 2.98675 × 10−10 | 1.000 | 0.872327 |
| U | 92 | 146 | 238 | 0.116 | 2.817 × 10−15 | 1.38 × 10−10 | 1.93281 × 10−21 | 5.45457 × 10−22 | 1.07027 × 10−11 | 2.54347826 | 5.8977 × 10−16 | 1.23951 × 10−15 | 7.678 × 10−16 | 9.25071 × 10−14 | 9.5212 × 10−14 | 6.94089 × 10−8 | 2.90419 × 10−9 | 4.16692 × 10−10 | 1.000 | 0.87452371 |
| ELEMENT | A | Entropy | |||||
|---|---|---|---|---|---|---|---|
| A | S(0,83) | S(0,84) | S(0,85) | S(0,86) | S(0,87) | S(0,88) | |
| Na | 23 | 5.58 × 10−9 | 5.67 × 10−9 | 5.75 × 10−9 | 5.83 × 10−9 | 5.92 × 10−9 | 6.01 × 10−9 |
| Mg | 24 | 9.59 × 10−9 | 9.73 × 10−9 | 9.88 × 10−9 | 1.00 × 10−8 | 1.02 × 10−8 | 1.03 × 10−8 |
| Al | 27 | 1.03 × 10−8 | 1.04 × 10−8 | 1.06 × 10−8 | 1.07 × 10−8 | 1.09 × 10−8 | 1.11 × 10−8 |
| Si | 28 | 1.70 × 10−8 | 1.73 × 10−8 | 1.75 × 10−8 | 1.78 × 10−8 | 1.81 × 10−8 | 1.84 × 10−8 |
| P | 31 | 2.25 × 10−8 | 2.29 × 10−8 | 2.32 × 10−8 | 2.36 × 10−8 | 2.40 × 10−8 | 2.44 × 10−8 |
| S | 32 | 2.81 × 10−8 | 2.85 × 10−8 | 2.90 × 10−8 | 2.94 × 10−8 | 2.99 × 10−8 | 3.04 × 10−8 |
| Cl | 35 | 3.61 × 10−8 | 3.67 × 10−8 | 3.73 × 10−8 | 3.78 × 10−8 | 3.85 × 10−8 | 3.91 × 10−8 |
| K | 39 | 4.40 × 10−9 | 4.47 × 10−9 | 4.55 × 10−9 | 4.61 × 10−9 | 4.70 × 10−9 | 4.78 × 10−9 |
| Ca | 40 | 6.85 × 10−9 | 6.97 × 10−9 | 7.08 × 10−9 | 7.19 × 10−9 | 7.32 × 10−9 | 7.44 × 10−9 |
| Sc | 45 | 8.00 × 10−9 | 8.14 × 10−9 | 8.28 × 10−9 | 8.40 × 10−9 | 8.56 × 10−9 | 8.71 × 10−9 |
| Ti | 48 | 8.98 × 10−9 | 9.13 × 10−9 | 9.29 × 10−9 | 9.43 × 10−9 | 9.61 × 10−9 | 9.77 × 10−9 |
| V | 51 | 9.77 × 10−9 | 9.95 × 10−9 | 1.01 × 10−8 | 1.03 × 10−8 | 1.05 × 10−8 | 1.06 × 10−8 |
| Cr | 52 | 1.04 × 10−8 | 1.06 × 10−8 | 1.08 × 10−8 | 1.10 × 10−8 | 1.12 × 10−8 | 1.14 × 10−8 |
| Mn | 55 | 1.14 × 10−8 | 1.16 × 10−8 | 1.18 × 10−8 | 1.20 × 10−8 | 1.22 × 10−8 | 1.24 × 10−8 |
| Fe | 56 | 1.22 × 10−8 | 1.24 × 10−8 | 1.26 × 10−8 | 1.28 × 10−8 | 1.31 × 10−8 | 1.33 × 10−8 |
| Co | 59 | 1.31 × 10−8 | 1.34 × 10−8 | 1.36 × 10−8 | 1.38 × 10−8 | 1.41 × 10−8 | 1.43 × 10−8 |
| Ni | 59 | 1.36 × 10−8 | 1.39 × 10−8 | 1.41 × 10−8 | 1.43 × 10−8 | 1.46 × 10−8 | 1.49 × 10−8 |
| Cu | 64 | 1.49 × 10−8 | 1.52 × 10−8 | 1.55 × 10−8 | 1.57 × 10−8 | 1.60 × 10−8 | 1.63 × 10−8 |
| Zn | 65 | 1.58 × 10−8 | 1.61 × 10−8 | 1.63 × 10−8 | 1.66 × 10−8 | 1.69 × 10−8 | 1.72 × 10−8 |
| Ga | 70 | 1.79 × 10−8 | 1.83 × 10−8 | 1.86 × 10−8 | 1.89 × 10−8 | 1.93 × 10−8 | 1.96 × 10−8 |
| Ge | 73 | 1.46 × 10−8 | 1.49 × 10−8 | 1.51 × 10−8 | 1.54 × 10−8 | 1.57 × 10−8 | 1.60 × 10−8 |
| As | 75 | 2.57 × 10−8 | 2.62 × 10−8 | 2.66 × 10−8 | 2.71 × 10−8 | 2.76 × 10−8 | 2.81 × 10−8 |
| Se | 79 | 3.20 × 10−8 | 3.26 × 10−8 | 3.32 × 10−8 | 3.37 × 10−8 | 3.44 × 10−8 | 3.51 × 10−8 |
| Br | 80 | 1.92 × 10−8 | 1.96 × 10−8 | 1.99 × 10−8 | 2.03 × 10−8 | 2.07 × 10−8 | 2.10 × 10−8 |
| Kr | 84 | 1.02 × 10−8 | 1.04 × 10−8 | 1.06 × 10−8 | 1.08 × 10−8 | 1.10 × 10−8 | 1.12 × 10−8 |
| Ru | 85 | 5.48 × 10−9 | 5.58 × 10−9 | 5.68 × 10−9 | 5.77 × 10−9 | 5.90 × 10−9 | 6.00 × 10−9 |
| Sr | 88 | 7.99 × 10−9 | 8.14 × 10−9 | 8.29 × 10−9 | 8.43 × 10−9 | 8.60 × 10−9 | 8.76 × 10−9 |
| Y | 89 | 8.57 × 10−9 | 8.73 × 10−9 | 8.89 × 10−9 | 9.04 × 10−9 | 9.23 × 10−9 | 9.40 × 10−9 |
| Zr | 91 | 9.17 × 10−9 | 9.35 × 10−9 | 9.52 × 10−9 | 9.68 × 10−9 | 9.88 × 10−9 | 1.01 × 10−8 |
| Nb | 93 | 9.99 × 10−9 | 1.02 × 10−8 | 1.04 × 10−8 | 1.05 × 10−8 | 1.08 × 10−8 | 1.10 × 10−8 |
| Mo | 96 | 1.10 × 10−8 | 1.12 × 10−8 | 1.14 × 10−8 | 1.16 × 10−8 | 1.18 × 10−8 | 1.21 × 10−8 |
| Tc | 99 | 1.20 × 10−8 | 1.22 × 10−8 | 1.25 × 10−8 | 1.27 × 10−8 | 1.29 × 10−8 | 1.32 × 10−8 |
| Ru | 101 | 5.98 × 10−9 | 6.09 × 10−9 | 6.21 × 10−9 | 6.31 × 10−9 | 6.45 × 10−9 | 6.57 × 10−9 |
| Rh | 103 | 2.22 × 10−8 | 2.27 × 10−8 | 2.31 × 10−8 | 2.35 × 10−8 | 2.40 × 10−8 | 2.44 × 10−8 |
| Pd | 108 | 1.46 × 10−8 | 1.49 × 10−8 | 1.52 × 10−8 | 1.54 × 10−8 | 1.58 × 10−8 | 1.61 × 10−8 |
| Ag | 108 | 1.53 × 10−8 | 1.56 × 10−8 | 1.59 × 10−8 | 1.62 × 10−8 | 1.65 × 10−8 | 1.68 × 10−8 |
| Cd | 112 | 1.64 × 10−8 | 1.67 × 10−8 | 1.71 × 10−8 | 1.73 × 10−8 | 1.77 × 10−8 | 1.80 × 10−8 |
| In | 115 | 1.76 × 10−8 | 1.80 × 10−8 | 1.83 × 10−8 | 1.86 × 10−8 | 1.90 × 10−8 | 1.94 × 10−8 |
| Sn | 119 | 2.06 × 10−8 | 2.10 × 10−8 | 2.14 × 10−8 | 2.18 × 10−8 | 2.23 × 10−8 | 2.27 × 10−8 |
| Sb | 122 | 2.47 × 10−8 | 2.52 × 10−8 | 2.56 × 10−8 | 2.61 × 10−8 | 2.66 × 10−8 | 2.71 × 10−8 |
| Te | 128 | 2.94 × 10−8 | 2.99 × 10−8 | 3.05 × 10−8 | 3.10 × 10−8 | 3.17 × 10−8 | 3.23 × 10−8 |
| I | 127 | 3.33 × 10−8 | 3.39 × 10−8 | 3.46 × 10−8 | 3.52 × 10−8 | 3.59 × 10−8 | 3.66 × 10−8 |
| Xe | 131 | 3.82 × 10−8 | 3.90 × 10−8 | 3.97 × 10−8 | 4.04 × 10−8 | 4.13 × 10−8 | 4.21 × 10−8 |
| Cs | 133 | 5.53 × 10−9 | 5.64 × 10−9 | 5.75 × 10−9 | 5.85 × 10−9 | 5.97 × 10−9 | 6.09 × 10−9 |
| Ba | 137 | 7.70 × 10−9 | 7.85 × 10−9 | 8.01 × 10−9 | 8.14 × 10−9 | 8.32 × 10−9 | 8.48 × 10−9 |
| La | 139 | 1.28 × 10−8 | 1.30 × 10−8 | 1.33 × 10−8 | 1.35 × 10−8 | 1.38 × 10−8 | 1.40 × 10−8 |
| Ce | 140 | 5.69 × 10−9 | 5.80 × 10−9 | 5.92 × 10−9 | 6.02 × 10−9 | 6.15 × 10−9 | 6.27 × 10−9 |
| Pr | 141 | 8.18 × 10−9 | 8.34 × 10−9 | 8.50 × 10−9 | 8.65 × 10−9 | 8.84 × 10−9 | 9.01 × 10−9 |
| Nd | 144 | 1.17 × 10−8 | 1.20 × 10−8 | 1.22 × 10−8 | 1.24 × 10−8 | 1.27 × 10−8 | 1.29 × 10−8 |
| Pm | 145 | 1.19 × 10−8 | 1.21 × 10−8 | 1.23 × 10−8 | 1.25 × 10−8 | 1.28 × 10−8 | 1.31 × 10−8 |
| Sm | 150 | 7.74 × 10−9 | 7.89 × 10−9 | 8.05 × 10−9 | 8.19 × 10−9 | 8.37 × 10−9 | 8.53 × 10−9 |
| Eu | 152 | 9.69 × 10−9 | 9.88 × 10−9 | 1.01 × 10−8 | 1.02 × 10−8 | 1.05 × 10−8 | 1.07 × 10−8 |
| Gd | 157 | 9.71 × 10−9 | 9.91 × 10−9 | 1.01 × 10−8 | 1.03 × 10−8 | 1.05 × 10−8 | 1.07 × 10−8 |
| Tb | 159 | 1.69 × 10−8 | 1.72 × 10−8 | 1.76 × 10−8 | 1.79 × 10−8 | 1.83 × 10−8 | 1.86 × 10−8 |
| Dy | 163 | 1.67 × 10−8 | 1.71 × 10−8 | 1.74 × 10−8 | 1.77 × 10−8 | 1.81 × 10−8 | 1.84 × 10−8 |
| Ho | 165 | 8.92 × 10−9 | 9.10 × 10−9 | 9.28 × 10−9 | 9.44 × 10−9 | 9.65 × 10−9 | 9.83 × 10−9 |
| Er | 167 | 1.07 × 10−8 | 1.09 × 10−8 | 1.11 × 10−8 | 1.13 × 10−8 | 1.15 × 10−8 | 1.17 × 10−8 |
| Tl | 204 | 2.05 × 10−8 | 2.09 × 10−8 | 2.14 × 10−8 | 2.17 × 10−8 | 2.22 × 10−8 | 2.27 × 10−8 |
| Yb | 173 | 1.12 × 10−8 | 1.15 × 10−8 | 1.17 × 10−8 | 1.19 × 10−8 | 1.22 × 10−8 | 1.24 × 10−8 |
| Lu | 175 | 1.18 × 10−8 | 1.20 × 10−8 | 1.23 × 10−8 | 1.25 × 10−8 | 1.28 × 10−8 | 1.30 × 10−8 |
| Ta | 181 | 1.41 × 10−8 | 1.43 × 10−8 | 1.46 × 10−8 | 1.49 × 10−8 | 1.52 × 10−8 | 1.55 × 10−8 |
| W | 184 | 1.52 × 10−8 | 1.55 × 10−8 | 1.58 × 10−8 | 1.61 × 10−8 | 1.64 × 10−8 | 1.68 × 10−8 |
| Re | 186 | 2.94 × 10−8 | 3.00 × 10−8 | 3.06 × 10−8 | 3.11 × 10−8 | 3.18 × 10−8 | 3.25 × 10−8 |
| Os | 190 | 1.68 × 10−8 | 1.71 × 10−8 | 1.75 × 10−8 | 1.78 × 10−8 | 1.82 × 10−8 | 1.85 × 10−8 |
| Ir | 192 | 2.99 × 10−8 | 3.06 × 10−8 | 3.12 × 10−8 | 3.17 × 10−8 | 3.24 × 10−8 | 3.30 × 10−8 |
| Pt | 195 | 2.94 × 10−8 | 3.00 × 10−8 | 3.06 × 10−8 | 3.11 × 10−8 | 3.18 × 10−8 | 3.24 × 10−8 |
| Au | 197 | 1.92 × 10−8 | 1.96 × 10−8 | 2.00 × 10−8 | 2.04 × 10−8 | 2.08 × 10−8 | 2.12 × 10−8 |
| Hg | 201 | 2.01 × 10−8 | 2.05 × 10−8 | 2.09 × 10−8 | 2.13 × 10−8 | 2.18 × 10−8 | 2.22 × 10−8 |
| Tl | 204 | 2.05 × 10−8 | 2.09 × 10−8 | 2.14 × 10−8 | 2.17 × 10−8 | 2.22 × 10−8 | 2.27 × 10−8 |
| Pb | 207 | 2.50 × 10−8 | 2.55 × 10−8 | 2.60 × 10−8 | 2.65 × 10−8 | 2.71 × 10−8 | 2.76 × 10−8 |
| Bi | 209 | 2.89 × 10−8 | 2.95 × 10−8 | 3.01 × 10−8 | 3.06 × 10−8 | 3.13 × 10−8 | 3.19 × 10−8 |
| Po | 210 | 3.24 × 10−8 | 3.30 × 10−8 | 3.37 × 10−8 | 3.43 × 10−8 | 3.50 × 10−8 | 3.57 × 10−8 |
| At | 210 | 3.64 × 10−8 | 3.71 × 10−8 | 3.79 × 10−8 | 3.85 × 10−8 | 3.94 × 10−8 | 4.02 × 10−8 |
| Rn | 222 | 4.18 × 10−8 | 4.27 × 10−8 | 4.35 × 10−8 | 4.43 × 10−8 | 4.53 × 10−8 | 4.62 × 10−8 |
| Fr | 223 | 8.91 × 10−9 | 9.09 × 10−9 | 9.27 × 10−9 | 9.44 × 10−9 | 9.65 × 10−9 | 9.84 × 10−9 |
| Ra | 226 | 1.39 × 10−8 | 1.42 × 10−8 | 1.44 × 10−8 | 1.47 × 10−8 | 1.50 × 10−8 | 1.53 × 10−8 |
| Ac | 227 | 1.68 × 10−8 | 1.71 × 10−8 | 1.75 × 10−8 | 1.78 × 10−8 | 1.82 × 10−8 | 1.85 × 10−8 |
| Th | 232 | 2.00 × 10−8 | 2.04 × 10−8 | 2.08 × 10−8 | 2.12 × 10−8 | 2.17 × 10−8 | 2.21 × 10−8 |
| Pa | 231 | 2.38 × 10−8 | 2.43 × 10−8 | 2.48 × 10−8 | 2.53 × 10−8 | 2.58 × 10−8 | 2.63 × 10−8 |
| U | 238 | 3.33 × 10−8 | 3.40 × 10−8 | 3.47 × 10−8 | 3.53 × 10−8 | 3.61 × 10−8 | 3.68 × 10−8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, E.; Recalde, N.; Chacón, E.J. Extraction of the Proton and Electron Radii from Characteristic Atomic Lines and Entropy Principles. Entropy 2017, 19, 293. https://doi.org/10.3390/e19070293
Jiménez E, Recalde N, Chacón EJ. Extraction of the Proton and Electron Radii from Characteristic Atomic Lines and Entropy Principles. Entropy. 2017; 19(7):293. https://doi.org/10.3390/e19070293
Chicago/Turabian StyleJiménez, Edward, Nicolás Recalde, and Esteban Jiménez Chacón. 2017. "Extraction of the Proton and Electron Radii from Characteristic Atomic Lines and Entropy Principles" Entropy 19, no. 7: 293. https://doi.org/10.3390/e19070293






