Biological Water Dynamics and Entropy: A Biophysical Origin of Cancer and Other Diseases †
Abstract
:1. Introduction: Is Biomacromolecular Dysfunction a Cause or Biomarker of Disease?
2. Historical Background: Advances in Measurement of Biologically Relevant Water Properties
3. Central Thesis: EIWS Drives Extracellular and Intracellular Changes toward Disease
- (a)
- Life-enabling water structures in the aqueous interphase, normally maintained by weak magnetic fields and the heparan sulfate proteoglycans (HSPGs) that decorate cell membrane surfaces, are disrupted by exogenous interfacial water stressors such as aluminum cations.
- (b)
- This disruption leads to localized water hydrophobicity, unwetting, increased water tension, and membrane “softening.” In addition, cationic aluminum ties up cell surface HSPGs by charge neutralization and thus breaks up the HSPG-membrane complex that connects extracellular matrix components to the intracellular cytoskeleton.
- (c)
- The resulting disconnection of the cytoskeleton from the plasma membrane has several adverse consequences, including impaired electrical conductivity of the cytoskeleton and microtubules and re-orientation of the cytoskeleton toward the cell nucleus, which can accelerate the pathological mitosis characteristic of cancer.
- (d)
- In addition, penetration of the interfacial water stressor (e.g., aluminum cations) into the cell disrupts intracellular water structure, leading to unfolded protein response, unfolded DNA response, and excess ROS production.
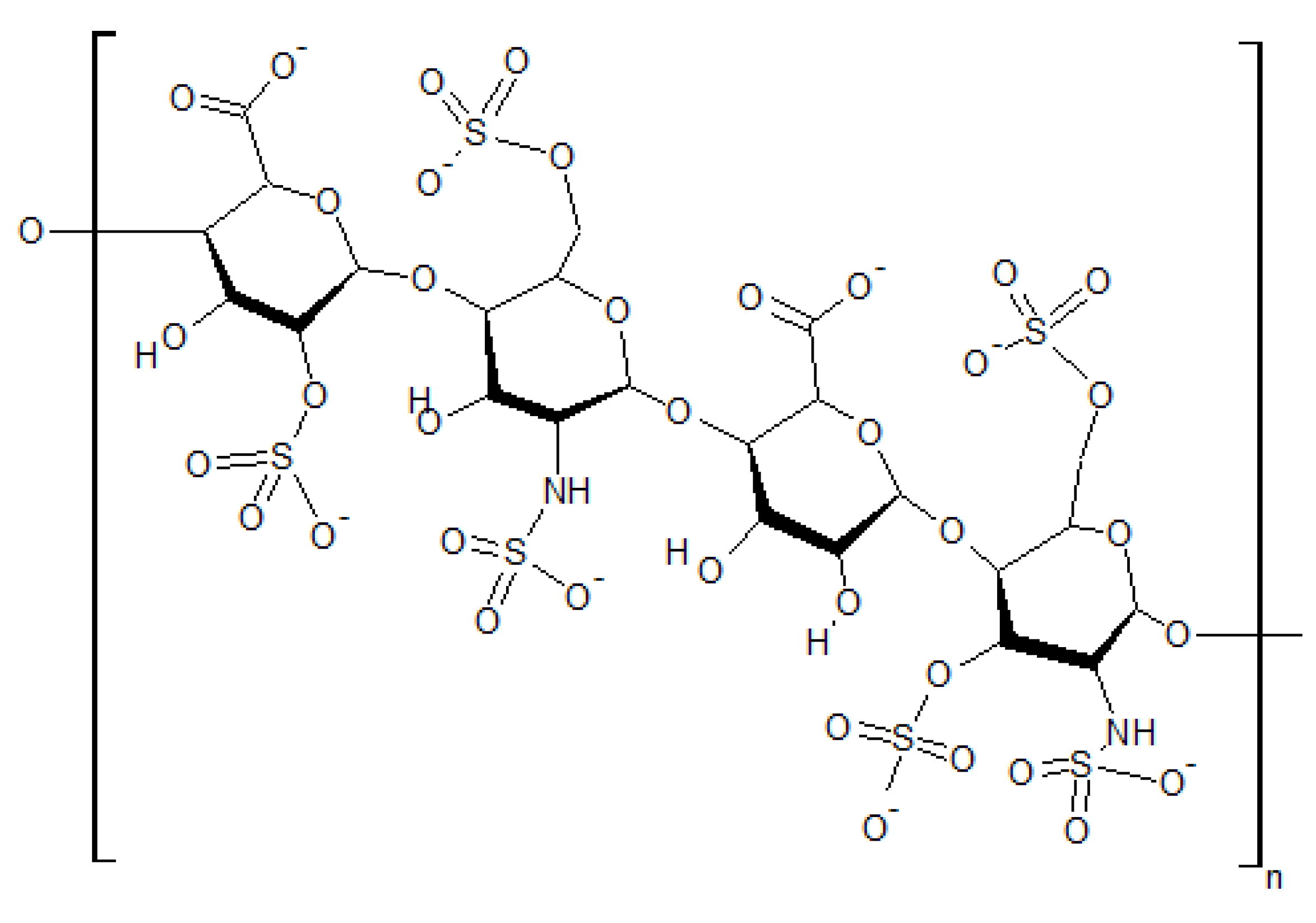
4. Biological Water Structures in Extracellular and Intracellular Space
4.1. Interaction with Small Solutes
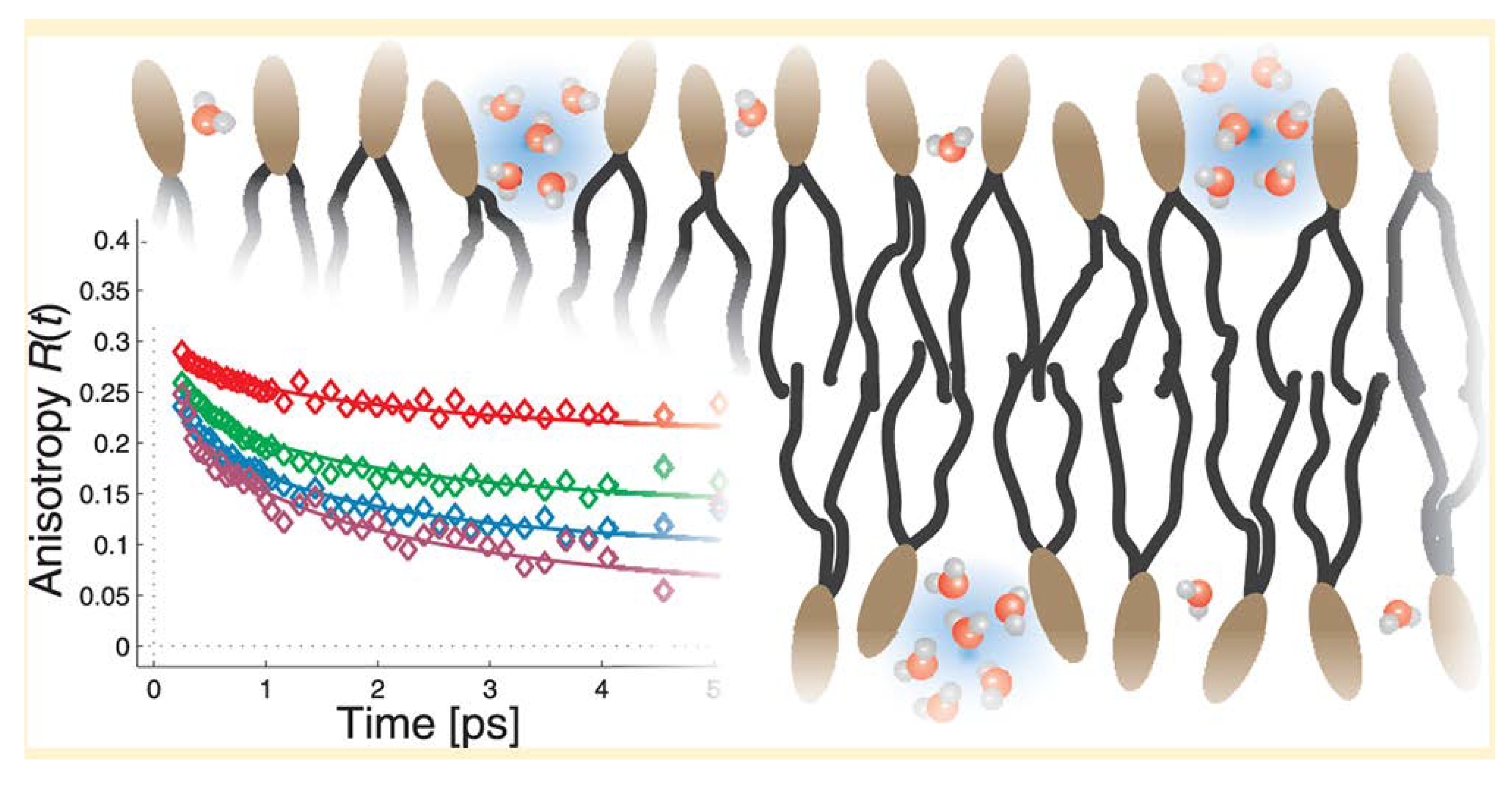
4.2. Interfacial Water: Interaction with Hydrophilic and Hydrophobic Surfaces
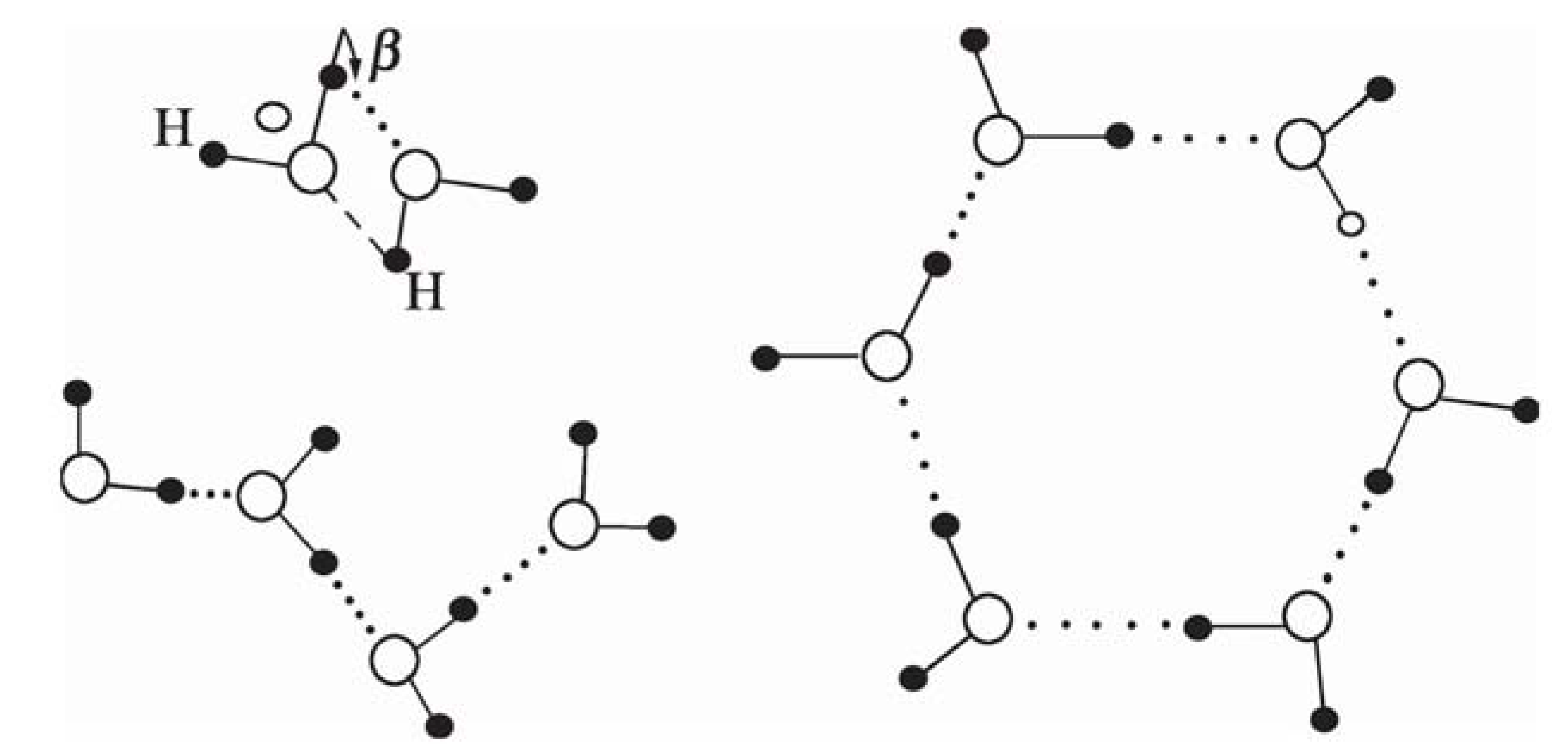
4.3. Interaction with Electric and Magnetic Fields
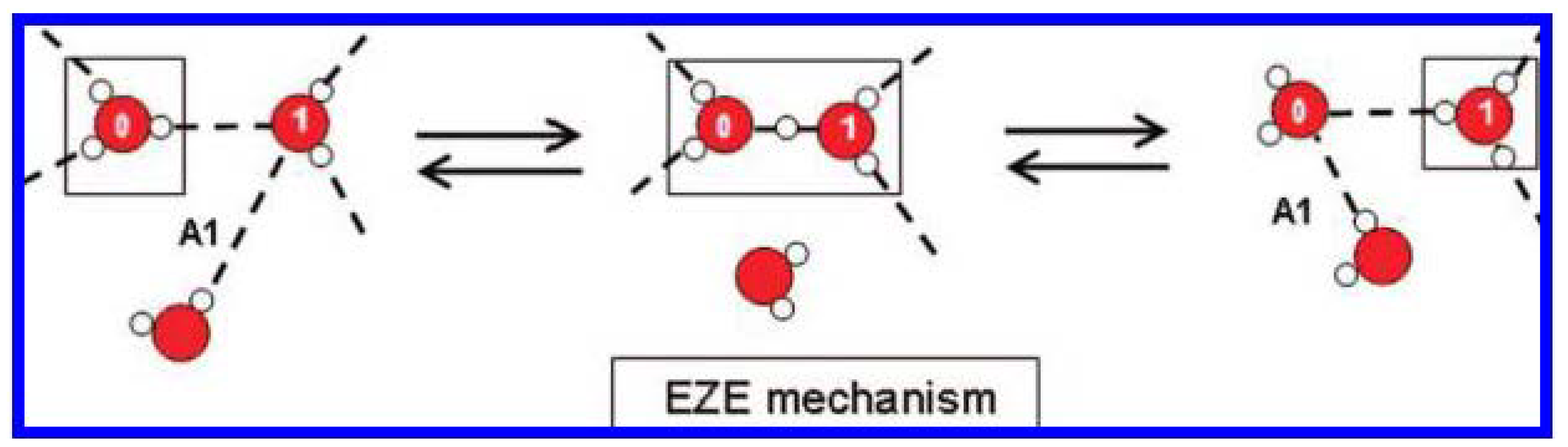
4.4. Life-Enabling Properties of Water at the Interphase
- (A)
- Promoting electrical conductivity at biological interfaces, thereby facilitating metabolism and voltage differences maintained by intracellular organelles;
- (B)
- Absorbing, storing, and emitting electromagnetic energy, enabling storage and transmission of energy and information;
- (C)
- Overcoming the kT or “thermal diffusion” problem; and
- (D)
- Solving the intracellular crowding and molecular self-assembly problems by way of chirality (handedness of molecules) and magnetization.
4.4.1. Promoting Electrical Conductivity at Biological Interfaces
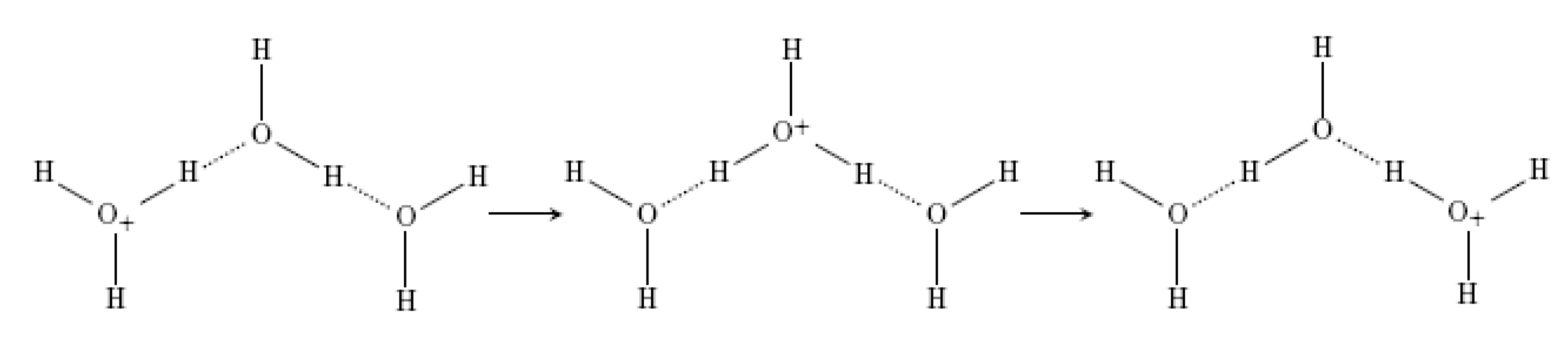
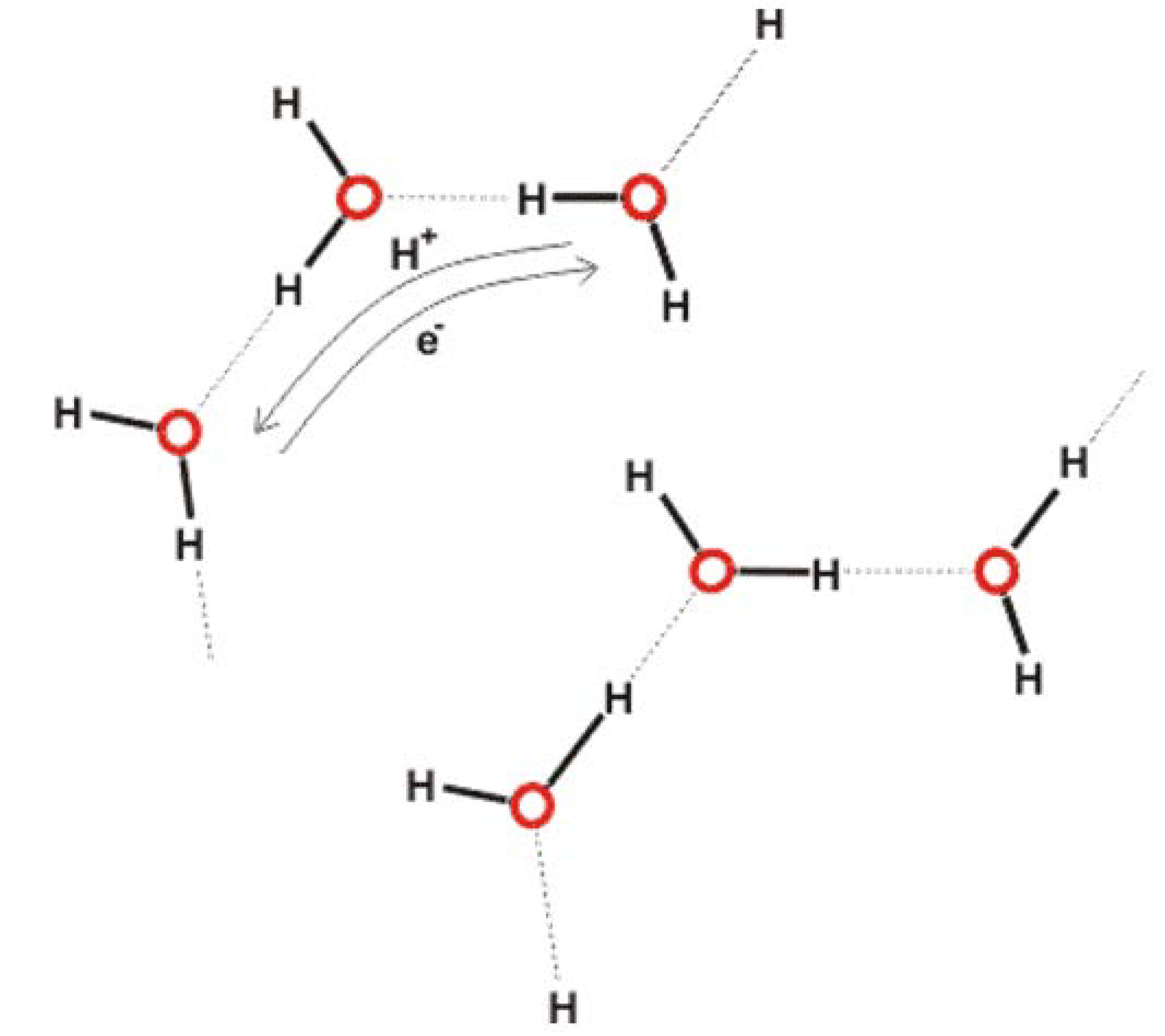
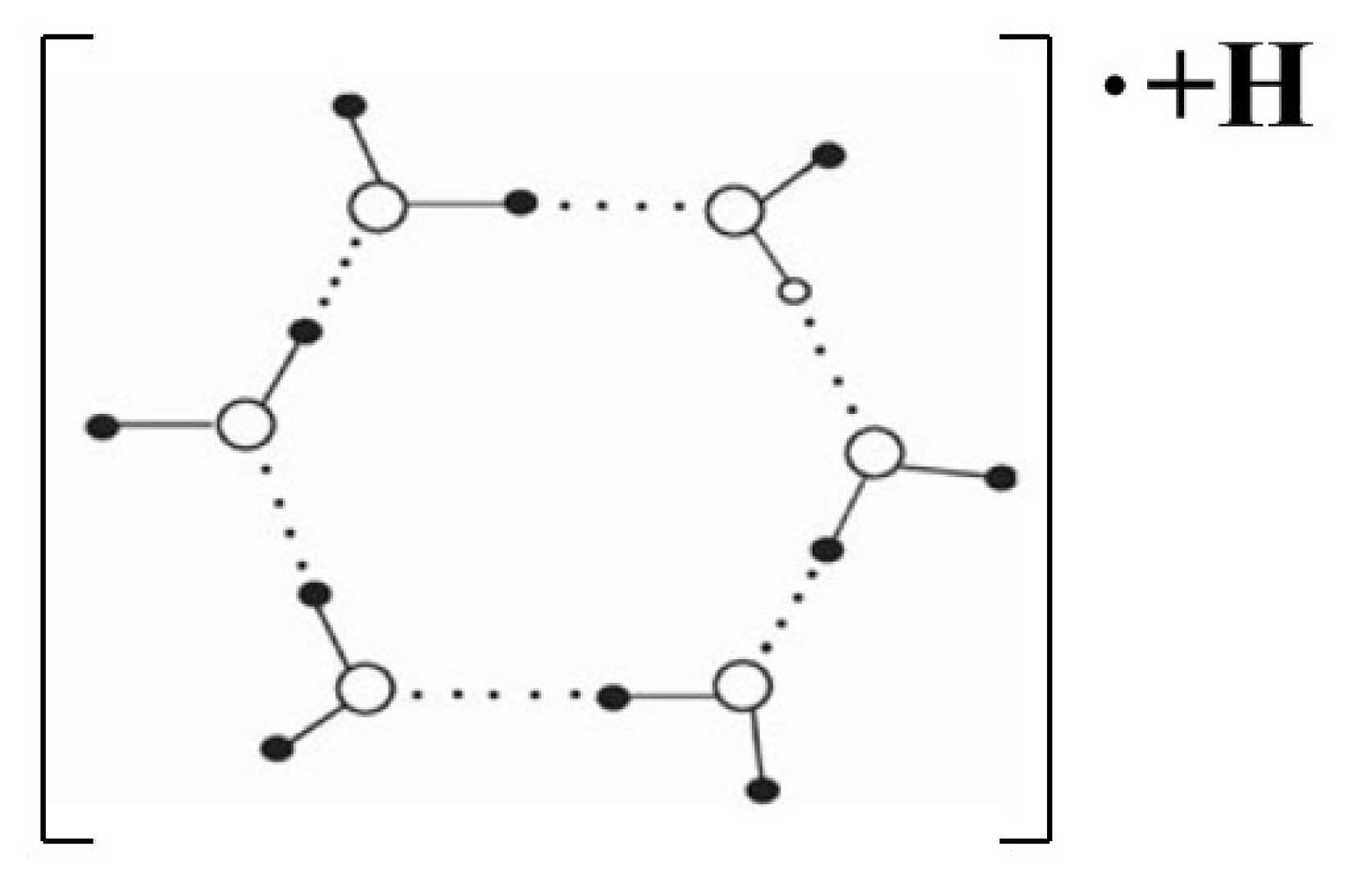
4.4.2. Absorbing, Storing, and Emitting Electromagnetic Energy
4.4.3. Overcoming the kT or “Thermal Diffusion” Problem
4.4.4. Solving the Intracellular Crowding and Molecular Self-Assembly Problems by Way of Chirality and Magnetization

4.5. Tuning the Aqueous Interphase: Modulating Interfacial Water Systems
5. Exogenous Interfacial Water Stress and Its Pathological Consequences
5.1. Exogenous Interfacial Water Stress as a Short-Circuit, Energy-Unloading Phenomenon Causing Extracellular and/or Intracellular Damage
5.2. Al3+ as a Biosignaling Nightmare
5.2.1. Displacement of Endogenous Cations
5.2.2. Reduction of Sulfur Bioavailability
5.2.3. Coagulant Action: Relevant Observations from Water Purification Chemistry
5.2.4. Induction of Oxidative, Genotoxic, and Protein Conformational Stress
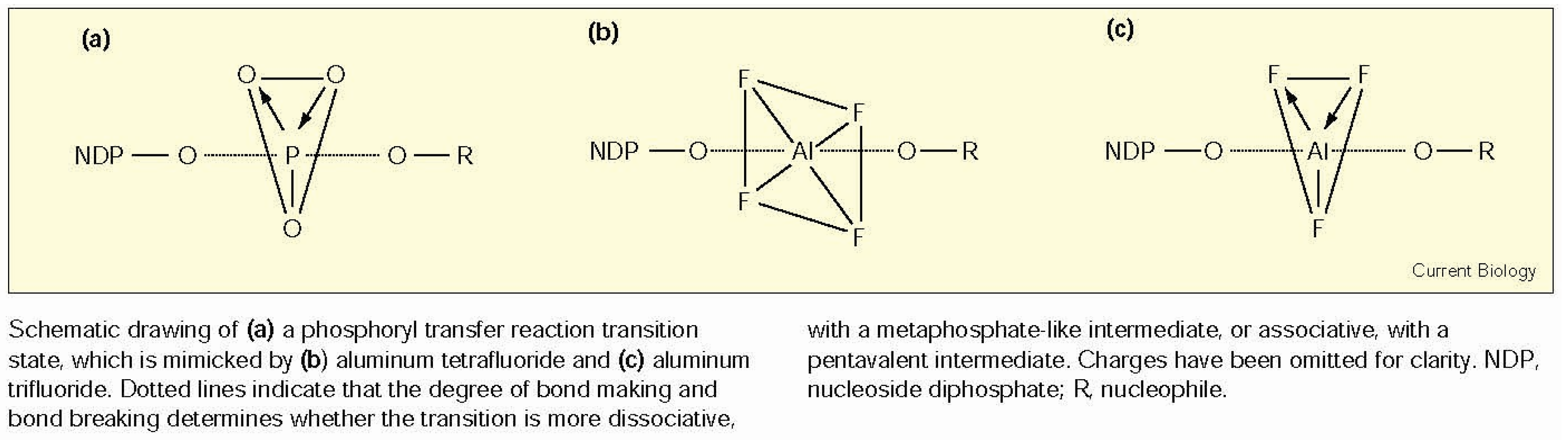
5.2.5. Induction of Interfacial Water Stress
6. Application of EIWS to Specific Pathologies
6.1. Breast Cancer
6.2. Neurological Disease
6.3. Infectious Disease
6.4. EIWS and Disease
7. Conclusions
Acknowledgments
Conflicts of interest
References
- Oller, J.W. The antithesis of entropy: Biosemiotic communication from genetics to human language with special emphasis on the immune systems. Entropy 2010, 12, 631–705. [Google Scholar] [CrossRef]
- Davidson, R.M.; Seneff, S. The initial common pathway of inflammation, disease, and sudden death. Entropy 2012, 14, 1399–1442. [Google Scholar] [CrossRef] [Green Version]
- Oschman, J.L. Energy Medicine: The Scientific Basis; Churchill Livingston: New York, NY, USA, 2000. [Google Scholar]
- Sternickel, K.; Braginski, A.I. Biomagnetism using SQUIDs: status and perspectives. Supercond. Sci. Technol. 2006, S160–S171. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 2005, 191, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Damadian, R.V. Apparatus and method for detecting cancer in tissue. U.S. Patent Number 3,789,832, 4 February 1974. [Google Scholar]
- Pollack, G. Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Function; Ebner & Sons: Seattle, WA, USA, 2001. [Google Scholar]
- Damadian, R. Tumor detection by nuclear magnetic resonance. Science 1971, 171, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Beall, P.T.; Brinkley, B.R.; Chang, D.C.; Hazlewood, C.F. Microtubule complexes correlated with growth rate and water proton relaxation times in human breast cancer cells. Cancer Res. 1982, 42, 4124–4130. [Google Scholar] [CrossRef]
- Cameron, I.L.; Ord, V.A.; Fullerton, G.D. Characterization of proton NMR relaxation times in normal and pathological tissues by correlation with other tissue parameters. Magn. Reson. Imaging 1984, 2, 97–106. [Google Scholar] [CrossRef]
- Cameron, I.L.; Hunter, K.E.; Ord, V.A.; Fullerton, G.D. Relationships between ice crystal size, water content and proton NMR relaxation times in cells. Physiol. Chem. Phys. Med. NMR 1985, 17, 371–386. [Google Scholar] [PubMed]
- Cameron, I.L.; Cook, K.R.; Edwards, D.; Fullerton, G.D.; Schatten, G.; Schatten, H.; Zimmerman, A.M.; Zimmerman, S. Cell cycle changes in water properties in sea urchin eggs. J. Cell. Physiol. 1987, 133, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.C.; Ruffle, S.V.; Ramirez-Cuesta, A.J.; Michararias, I.; Beta, I.; Miller, A.; Li, J. Inelastic incoherent neutron scattering measurements of intact cells and tissues and detection of interfacial water. J. Am. Chem. Soc. 2004, 126, 4682–4688. [Google Scholar] [CrossRef] [PubMed]
- Mamontov, E.; Chu, X.-Q. Water-protein dynamic coupling and new opportunities for probing it at low to physiological temperatures in aqueous solutions. Phys. Chem. Chem. Phys. 2012, 14, 11573–11588. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Z.; Yan, E.C.Y. Chiral Vibrational Structures of Proteins at Interfaces Probed by Sum Frequency Generation Spectroscopy. Int. J. Mol. Sci. 2011, 12, 9404–9425. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Prell, J.S.; Bush, M.F.; Williams, E.R. Sulfate ion patterns water at long distance. J. Am. Chem. Soc. 2010, 132, 8248–8249. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, H.; Chen, G.; Berendzen, J.; Fenimore, P.W.; Jansson, H.; McMahon, B.H.; Stroe, I.R.; Swenson, J.; Young, R.D. A unified model of protein dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Born, B.; Havenith, M.; Gruebele, M. Real-time detection of protein-water dynamics upon protein folding by terahertz absorption spectroscopy. Angew. Chem. Int. Ed. 2008, 47, 6486–6489. [Google Scholar] [CrossRef] [PubMed]
- Heyden, M.; Ebbinghaus, S.; Havenith, M. Terahertz spectroscopy as a tool to study hydration dynamics. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2006; pp. 1–19. [Google Scholar]
- Heyden, M.; Sun, J.; Funkner, S.; Mathias, G.; Forbert, H.; Havenith, M.; Marx, D. Dissecting the THz spectrum of liquid water from first principles via correlations in time and space. Proc. Natl. Acad. Sci. USA 2010, 107, 12068–12073. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, S.; Meister, K.; Born, B.; DeVries, A.L.; Gruebele, M.; Havenith, M. Antifreeze glycoprotein activity correlates with long-range protein-water dynamics. J. Am. Chem. Soc. 2010, 132, 12210–12211. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.Q.; Verma, P.K.; Mitra, R.K.; Havenith, M. Do hydration dynamics follow the structural perturbation during thermal denaturation of a protein: A terahertz absorption study. Biophys. J. 2011, 101, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Born, B.; Heyden, M.; Tworowski, D.; Fields, G.B.; Sagi, I.; Havenith, M. Correlated structural kinetics and retarded solvent dynamics at the metalloprotease active site. Nat. Struct. Mol. Biol. 2011, 18, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Heugen, U.; Schwaab, G.; Brundermann, E.; Heyden, M.; Yu, X.; Leitner, D.M.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Havenith, M. Watching the dance of water in the hydration shell of ions and biomolecules in the THz frequency range. Keynote Lecture. In Proceedings of 86th Am. Chem. Soc. Colloid & Surface Science Symposium, Johns-Hopkins University, Baltimore, MD, USA, 11 June 2012.
- Jansson, H.N.; Bergman, R.; Swenson, J. Role of solvent for the dynamics and the glass transition of proteins. J. Phys. Chem. B 2011, 115, 4099–4109. [Google Scholar] [CrossRef] [PubMed]
- Sterpone, F.; Stirnemann, G.; Laage, D. Magnitude and molecular origin of water slowdown next to a protein. J. Am. Chem. Soc. 2012, 134, 4116–4119. [Google Scholar] [CrossRef] [PubMed]
- Feig, M.; Sugita, Y. Variable interactions between protein crowders and biomolecular solutes are important in understanding cellular crowding. J. Phys. Chem. B 2011, 116, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Sugita, Y.; Feig, M. Protein crowding affects hydration structure and dynamics. J. Am. Chem. Soc. 2012, 134, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Miklos, A.C.; Sarkar, M.; Wang, Y.; Pielak, G.J. Protein crowding tunes protein stability. J. Am. Chem. Soc. 2011, 133, 7116–7120. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, A.P.; Wang, Y.; Tadeo, X.; Millet, O.; Pielak, G.J. Macromolecular crowding fails to fold a globular protein in cells. J. Am. Chem. Soc. 2011, 133, 8082–8085. [Google Scholar] [CrossRef] [PubMed]
- Combet, S.; Zanotti, J.-M. Further evidence that interfacial water is the main “driving force” of protein dynamics: A neutron scattering study on perdeuterated C-phycocyanin. Phys. Chem. Chem. Phys. 2012, 14, 4927–4934. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, S.E.; Cerveny, S.; Alegria, A.; Colmenero, J. The dynamical behavior of hydrated glutathione: a model for protein-water interactions. Phys. Chem. Chem. Phys. 2010, 12, 10512–10517. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Arteche, I.; Cerveny, S.; Alegria, A.; Colmenero, J. Dielectric spectroscopy in the GHz region on fully hydrated zwitterionic amino acids. Phys. Chem. Chem. Phys. 2012, 14, 11352–11362. [Google Scholar] [CrossRef] [PubMed]
- Gruebele, M. Biological water: A flexible designer fluid? Water Conditioning & Purification 2009, 51, 2. http://www.wcponline.com/TOC.cfm?ISN=140 (accessed on Sep. 9, 2013). [Google Scholar]
- Chen, S.-H.; Lagii, M.; Chu, X.-Q.; Zhang, Y.; Kim, C.; Faraone, A.; Fratini, E.; Baglioni, S. Dynamics of a globular protein and its hydration water studied by neutron scattering and MD simulations. Spectroscopy 2010, 1–24. [Google Scholar] [CrossRef]
- Khodadadi, S.; Roh, J.H.; Kisluik, A.; Mamontov, E.; Tyagi, M.; Woodson, S.A.; Briber, R.M.; Solokov, A.P. Dynamics of biological macromolecules: Not a simple slaving by hydration water. Biophys. J. 2010, 98, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Fuxreiter, M.; Mezei, M.; Simon, I.; Osmany, R. Interfacial water as a “hydration fingerprint” in the noncognate complex of BamHI. Biophys. J. 2005, 89, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Geckeler, K.E.; Rupp, F.; Geis-Gerstorfer, J. Interfaces and interphases of (bio)materials: Definitions, structures, and dynamics. Adv. Mater. 1997, 9, 513–518. [Google Scholar] [CrossRef]
- Hofmeister, F. Naunyn-Schmiedebergs Zur Lehre von der Wirkung der Salze (Article in German). Arch. Pharmacol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D.; Neilson, G.W.; Enderby, J.E. Ions in water: Characterizing forces that control chemical processes and physical structure. Biophys. J. 2007, 128, 95–104. [Google Scholar]
- Lo Nostro, P.; Ninham, B.W. Hofmeister phenomena: An update on ion specificity in biology. Chem. Rev. 2012, 112, 2286–2322. [Google Scholar] [PubMed]
- Barnes, F.S. Interaction of direct current and extremely low-frequency electric fields with biological materials and systems. In Bioengineering and Biophysical Aspects of Electromagnetic Fields; Barnes, F., Greenebaum, B., Eds.; CRC Press: Boca Raton, FL, USA, 2006; p. 120. [Google Scholar]
- Sietze Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.; Chandler, D.; Weeks, J.D. Hydrophobicity at small and large length scales. J. Phys. Chem. 1999, 103, 4570–4577. [Google Scholar] [CrossRef]
- Zangi, R. Driving force for hydrophobic interaction at different length scales. J. Phys. Chem. B 2011, 115, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Goertz, M.P.; Houston, J.E.; Zhu, X.-Y. Hydrophilicity and the viscosity of interfacial water. Langmuir 2007, 23, 5491–5497. [Google Scholar] [CrossRef] [PubMed]
- Sendner, C.; Horinek, D.; Bocquet, L.; Netz, R.R. Interfacial water at hydrophobic and hydrophilic surfaces: Slip, viscosity, and diffusion. Langmuir 2009, 25, 10768–10781. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.P.; Hodeck, K.F.; Zhu, D.; Kothe, A.; Lange, K.M.; Fecht, H.-J.; Aziz, E.F. Breathing volume into interfacial water with laser light. J. Phys. Chem. Lett. 2011, 2, 562–565. [Google Scholar] [CrossRef]
- Huang, D.M.; Chandler, D. Temperature and length scale dependence of hydrophobic effects and their possible implications for protein folding. Proc. Natl. Acad. Sci. USA 2000, 97, 8324–8327. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Varilly, P.; Jamadagni, S.N.; Hagan, M.F.; Chandler, D.; Garde, S. Sitting at the edge: How biomolecules use hydrophobicity to tune their interactions and function. J. Phys. Chem. B 2012, 116, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Varilly, P.; Jamadagni, S.N.; Acharya, H.; Garde, S.; Chandler, D. Extended surfaces modulate hydrophobic interactions of neighboring solutes. Proc. Natl. Acad. Sci. USA 2011, 108, 17678–17683. [Google Scholar] [CrossRef] [PubMed]
- Sarupria, S.; Garde, S. Quantifying water density fluctuations and compressibility of hydration shells of hydrophobic solutes and proteins. Phys. Rev. Lett. 2009, 103, 037803. [Google Scholar] [CrossRef] [PubMed]
- Acharya, H.; Vembanur, S.; Jamadagni, S.N.; Garde, S. Mapping hydrophobicity at the nanoscale: Applications to heterogeneous surfaces and proteins. Faraday Discuss. 2010, 146, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Garde, S.; Patel, A.J. Unraveling the hydrophobic effect, one molecule at a time. Proc. Natl. Acad. Sci. USA 2011, 108, 16491–16492. [Google Scholar] [CrossRef] [PubMed]
- Rezus, Y.L.A.; Bakker, H.J. Observation of immobilized water molecules around hydrophobic groups. Phys. Rev. Lett. 2007, 99, 148301. [Google Scholar] [CrossRef] [PubMed]
- Despa, F.; Fernández, A.; Berry, R.S. Dielectric Modulation of Biological. Water. Phys. Rev. Lett. 2004, 93, 228104. [Google Scholar] [CrossRef] [PubMed]
- Despa, F. Biological water: Its vital role in macromolecular structure and function. Ann. NY Acad. Sci. 2005, 1066, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ashbaugh, H.S.; Asthagiri, D.; Pratt, L.R.; Rempe, S.B. Hydration of krypton and consideration of clathrate models of hydrophobic effects from the perspective of quasi-chemical theory. Biophys. Chem. 2003, 105, 323–338. [Google Scholar] [CrossRef]
- Liang, S.; Kusalik, P.G. Nucleation of gas hydrates within constant energy systems. J. Phys. Chem. B 2013, in press. [Google Scholar] [CrossRef] [PubMed]
- Blokzijl, W.; Engberts, J.B.F.N. Hydrophobic Effects. Opinions and Facts. Angewandte. Chemie. International Edition in English 1993, 32, 1545–1579. [Google Scholar] [CrossRef]
- Dixit, S.; Crain, J.; Poon, W.C.K.; Finney, J.L.; Soper, A.K. Molecular segregation observed in a concentrated alcohol-water solution. Nature 2002, 416, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Water as an Active Constituent in Cell Biology. Chemical Reviews 2007, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Keutsch, F.N.; Saykally, R.J. Water clusters: Untangling the mysteries of the liquid, one molecule at a time. Proc. Natl. Am. Sci. USA 2011, 98, 10533–10540. [Google Scholar] [CrossRef] [PubMed]
- Csajka, F.S.; Chandler, D. Transition pathways in a many-body system: Application to hydrogen-bond breaking in water. J. Chem. Phys. 1998, 109, 1125–1133. [Google Scholar] [CrossRef]
- Xantheas, S.S. Ab initio studies of cyclic water clusters (H2O)n, n = 1–6. III. Comparison of density functional with MP2 results. J. Chem. Phys. 1995, 102, 4505–4517. [Google Scholar] [CrossRef]
- Nauta, K.; Miller, R.E. Formation of cyclic water hexamer in liquid helium: The smallest piece of ice. Science 2000, 287, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Muckle, M.T.; Zaleski, D.P.; Seifert, N.A.; Temelso, B.; Shields, G.C.; Kisiel, Z.; Pate, B.H. Structures of cage, prism, and book isomers of water hexamer from broadband rotational spectroscopy. Science 2012, 336, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Piatkowski, L.; de Heij, J.; Bakker, H.J. Probing the distribution of water molecules hydrating lipid membranes with ultrafast forster vibrational energy transfer. J. Phys. Chem. B 2013, in press. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. The endothelial surface layer. Pflugers. Arch. EJP 2000, 440, 653–666. [Google Scholar] [CrossRef]
- Tabuchi, A.; Mertens, M.; Kuppe, H.; Pries, A.R.; Kuebler, W.M. Intravital microscopy of the murine pulmonary microcirculation. J. Appl. Physiol. 2008, 104, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.-H.; Zheng, J.-M.; Zhao, Q.; Pollack, G.H. Spectroscopic studies of solutes in aqueous solution. J. Phys. Chem. A 2008, 112, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Pollack, G.H.; Figueroa, X.; Zhao, Q. Review: Molecules, water, and radiant energy: New clues for the origin of life. Int. J. Mol. Sci. 2009, 10, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Yoo, H.; Pollack, G.H. Effect of Radiant Energy on Near-Surface Water. J. Phys. Chem. B 2009, 113, 13953–13958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.M.; Chin, W.C.; Khijniak, E.; Khijniak, E., Jr.; Pollack, G.H. Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv. Colloid Interface Sci. 2006, 127, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Spinetti, P.R.; Tedeschi, A. Water dynamics at the root of metamorphosis in living organisms. Water 2010, 2, 566–586. [Google Scholar] [CrossRef]
- Lo, S.Y.; Geng, X.; Gann, D. Evidence for the existence of stable-water-clusters at room temperature and normal pressure. Phys. Lett. A 2009, 373, 3872–3876. [Google Scholar] [CrossRef]
- Rai, D.; Kulkarni, A.D.; Gejji, S.P.; Pathak, R.K. Water clusters (H2O)n, n=6–8, in external electric fields. J. Chem. Phys. 2008, 128, 034310. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Gutiérrez, S.; Hernández-Rojas, J.; Bretón, J.; Llorente, J.M.; Wales, D.J. Physical properties of small water clusters in low and moderate electric fields. J. Chem. Phys. 2011, 135, 124303. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-T.; Weng, C.-I. The effect of an external magnetic field on the structure of liquid water using molecular dynamics simulation. J. App. Phys. 2006, 100, 043917. [Google Scholar] [CrossRef]
- Pang, X.; Deng, B. Investigation of changes in properties of water under the action of a magnetic field. Sci. China Ser. G-Phys. Mech. Astron. 2008, 51, 1621–1632. [Google Scholar] [CrossRef]
- Pang, X.-F.; Deng, B. The changes of macroscopic features and microscopic structures of water under influence of magnetic field. Physica. B 2008, 403, 3571–3577. [Google Scholar] [CrossRef]
- Pang, X.-F.; Deng, B. Infrared absorption spectra of pure and magnetized water at elevated temperatures. EPL 2010, 92, 65001. [Google Scholar] [CrossRef]
- Pang, X.-F.; Deng, B.; Tang, B. Influences of magnetic field on macroscopic properties of water. Mod. Phys. Lett. B 2012, 26, 1250069. [Google Scholar] [CrossRef]
- Pang, X.F. The conductivity properties of protons in ice and mechanism of magnetization of liquid water. Eur. Phys. J. B 2006, 49, 5–23. [Google Scholar] [CrossRef]
- Mohri, K.; Fukushima, M. Milligauss magnetic field triggering reliable self-organization of water with long-range ordered proton transport through cyclotron resonance. IEEE Trans. Magn. 2003, 39, 3328–3330. [Google Scholar] [CrossRef]
- Mohri, K.; Uchiyama, T. Detection of human microvibration transmitted along solid using pico-tesla magneto-impedance sensor. IEEJ TEEE 2010, 5, 378–379. [Google Scholar] [CrossRef]
- Fukushima, M.; Mohri, K.; Kataoka, T.; Matsumoto, M. Milli gauss pulsed magnetic field applied phosphate buffeted saline solution elevates intracellular Ca2+ level and stimulates phagocytic activity of human neutrophils. Trans. Magn. Soc. Japan 2002, 2, 15–18. [Google Scholar] [CrossRef]
- Fukushima, M.; Kataoka, T.; Sugiyama, N.; Mohri, K. Milligauss magnetic field applied pure water exert firefly luciferin-luciferase luminescence and induce intracellular calcium elevation of CHO cells without ATP. IEEE Trans. Magn. 2005, 41, 4188–4190. [Google Scholar] [CrossRef]
- Johansson, B.; Sukhotskaya, S. Allometric scaling behavior—A quantum dissipative state implies a reduction in thermal infrared emission and fractal ordering in distilled coherent water. Water 2012, 3, 100–121. [Google Scholar]
- Del Giudice, E.G.; Tedeschi, A.; Vitiello, G.; Voeikov, V. Coherent structures in liquid water close to hydrophilic surfaces. J. Phys. Conf. Ser. 2013, 442, 012028. [Google Scholar] [CrossRef]
- Jung, Y.; Marcus, R.A. On the nature of organic catalysis “on water”. J. Am. Chem. Soc. 2007, 129, 5492–5502. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Dey, K.K.; Muddana, H.S.; Tabouillot, T.; Ibele, M.E.; Butler, P.J.; Sen, A. Enzyme molecules as nanomotors. J. Am. Chem. Soc. 2013, 135, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jo, K.; Kounovsky, K.L.; Pablo, J.J.d.; Schwartz, D.C. Molecular propulsion: Chemical sensing and chemotaxis of DNA driven by RNA polymerase. J. Am. Chem. Soc. 2009, 131, 5722–5723. [Google Scholar] [CrossRef] [PubMed]
- Baraban, L.; Harazim, S.M.; Sanchez, S.; Schmidt, O.G. Chemotactic behavior of catalytic motors in microfluidic channels. Angewandte Chemie 2013, 125, 5662–5666. [Google Scholar] [CrossRef]
- Gartzke, J.; Lange, K. Cellular target of weak magnetic fields: Ionic conduction along actin filaments. American journal of physiology. Cell. Physiol. 2002, 283, C1333–C1346. [Google Scholar] [CrossRef] [PubMed]
- Lange, K. Fundamental role of microvilli in the main functions of differentiated cells. J. Cell Physiol. 2011, 226, 896–927. [Google Scholar] [CrossRef] [PubMed]
- Mundy, D.I.; Machleidt, T.; Ying, Y.-S.; Anderson, R.G.W.; Bloom, G.S. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 2002, 115, 4327–4339. [Google Scholar] [CrossRef] [PubMed]
- Collot, M.; Louvard, D.; Singer, S.J. Lysosomes are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc. Nat. Acad. Sci. USA 1984, 81, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.R.; Pon, L.A. Interactions of mitochondria with the actin cytoskeleton. Biochim. Biophys. Acta. 2006, 1763, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Agmon, N. Salt effect on transient proton transfer to solvent and microscopic proton mobility. Chem. Phys. Lett. 1995, 64, 161–195. [Google Scholar] [CrossRef]
- Markovitch, O.; Chen, H.; Izvekov, S.; Paesani, F.; Voth, G.A.; Agmon, N. Special pair dance and partner selection: Elementary steps in proton transport in liquid water. J. Phys. Chem. B 2008, 112, 9456–9466. [Google Scholar] [CrossRef] [PubMed]
- Verdel, N.; Jerman, I.; Bukovec, P. The “Autothixotropic” phenomenon of water and its role in proton transfer. Int. J. Mol. Sci. 2011, 12, 7481–7494. [Google Scholar] [CrossRef] [PubMed]
- Verdel, N.; Jerman, I.; Krasovec, R.; Bukovec, P.; Zupancic, M. Possible time-dependent effect of ions and hydrophilic surfaces on the electrical conductivity of aqueous solutions. Int. J. Mol. Sci. 2012, 13, 4048–4068. [Google Scholar] [PubMed]
- Feng, S.; Voth, G.A. Proton solvation and transport in hydrated nafion. J. Phys. Chem. B 2011, 115, 5903–5912. [Google Scholar] [CrossRef] [PubMed]
- Jorn, R.; Voth, G.A. Mesoscale simulation of proton transport in proton exchange membranes. J. Phys. Chem. C 2012, 116, 10476–10489. [Google Scholar] [CrossRef]
- Zundel, G.; Metzer, H. Energiebänder der tunnelnden Überschuβ-Protonen in flüssigen Säuren. Eine IR-spektroskopische Untersuchung der Natur der Gruppierungen H5O2+. Z. Phys. Chem. 1968, 58, 225–245. (in German). [Google Scholar] [CrossRef]
- Knight, C.; Voth, G.A. The curious case of the hydrated proton. Acc. Chem. Res. 2012, 45, 101–109. [Google Scholar] [CrossRef]
- Habenicht, B.F.; Paddison, S.J. Ab initio simulations of the effects of nanoscale confinement on proton transfer in hydrophobic environments. J. Phys. Chem. B 2011, 115, 10826–10835. [Google Scholar]
- Martin Chaplin talks about the importance of water, in advance of the Colours of Water festival, March, 2013. http://www.i-sis.org.uk/coloursofwater/.
- Chaplin, M. What is Liquid Water? ISIS Report. Available online: http://www.i-sis.org.uk/What_is_Liquid_Water.php/ (accessed on 20 April 2013).
- Czerlinski, G.; Ypma, T. Stabilization of aqueous electromeric nano-domains. J. Comput. Theor. Nanos. 2011, 8, 1400–1408. [Google Scholar] [CrossRef]
- Czerlinski, G.; Ypma, T. Homeopathic potentization based on nanoscale domains. J. Altern. Complem. Med. 2011, 17, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Mizuse, K.; Kuo, J.-L.; Fujii, A. Structural trends of ionized water networks: Infrared spectroscopy of water cluster radical cations (H2O)n+ (n = 3–11). Chem. Sci. 2011, 2, 868–876. [Google Scholar] [CrossRef]
- Fröhlich, H. Bose condensation of strongly excited longitudinal electric modes. Phys. Lett. A 1968, 26, 402–403. [Google Scholar] [CrossRef]
- Fröhlich, H. Long-range coherence and energy storage in biological systems. Int. J. Quantum Chem. 1968, 2, 641–649. [Google Scholar] [CrossRef]
- Pokorný, J.; Foletti, A.; Kobilková, J.; Jandová, A.; Vrba, J.; Vrba, J., Jr.; Nedbalová, M.; Čoček, A.; Danani, A.; Tuszyński, J.A. Biophysical insights into cancer transformation and treatment. Sci. World J. 2013. Article ID 195028. [Google Scholar] [CrossRef]
- Pohl, H.A. Oscillating fields about growing cells. Int. J. Quant. Chem. 1980, 7, 411–431. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Mitsui, T.; Rose, M.K.; Fomin, E.; Ogletree, D.F.; Salmeron, M. Water Diffusion and Clustering on Pd(111). Science 2002, 297, 1850–1852. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.W. Fröhlich’s interpretation of biology through theoretical physics Chapter 7. In Herbert Fröhlich FRS: A physicist ahead of his time; Hyland, G.J., Rowlands, P., Eds.; The University of Liverpool: Liverpool, UK, 2006; pp. 91–138, (2nd edition 2008, pp.107–154). [Google Scholar]
- Ahmed, N.A.G.; Calderwood, J.H.; Fröhlich, H.; Smith, C.W. Evidence for collective magnetic effects in an enzyme: Likelihood of room temperature superconductive regions. Phys. Lett. 1975, 53A, 129–130. [Google Scholar] [CrossRef]
- Milani, M.; Del Giudice, E.; Doglia, S.; Vitiello, G.; Smith, C.W. Superconductive and Josephson-like behaviour of cells. La Radiologica. Medica.Radiol. Med. 1991, 81, 51–55. [Google Scholar]
- Stahler, J.; Bovensiepen, U.; Meyer, M.; Wolf, M. A surface science approach to ultrafast electron transfer and solvation dynamics at interfaces. Chem. Soc. Rev. 2008, 37, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-W.; Guan, Q.-F.; Zhu, Z.; Song, L.-T.; Yao, H.-B.; Lei, X.; Yu, S.-H. Highly conductive and stretchable conductors fabricated from bacterial cellulose. NPG Asia Mater. 2012, 4, e19. [Google Scholar] [CrossRef]
- Gascoyne, P.R.; Pethig, R.; Szent-Gyorgyi, A. Water structure-dependent charge transport in proteins. Proc. Natl. Acad. Sci. USA 1981, 78, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Careri, G.; Giansanti, A.; Rupley, J.A. Critical exponents of protonic percolation in hydrated lysozyme powders. Phys. Rev. A 1988, 37, 2703–2705. [Google Scholar] [CrossRef] [PubMed]
- Craddock, T.J.A.; Tuszynski, J.A.; Priel, A.; Freedman, H. Microtubule ionic conduction and its implications for higher cognitive functions. J. Integr. Neurosci. 2010, 9, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Polcari, A.; Romano, P.; Sabatino, L.; Del Vecchio, E.; Consales, M.; Cusano, A.; Cutolo, A.; Colantuoni, V. Electrical and optical characterization of DNA molecules as a function of concentration in aqueous solution. J. Appl. Phys. 2011, 109, 074703. [Google Scholar] [CrossRef]
- Sontz, P.A.; Muren, N.B.; Barton, J.K. DNA charge transport for sensing and signaling. Accounts Chem. Res. 2012, 45, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Careri, G.; Giansanti, A.; Rupley, J.A. Proton percolation on hydrated lysozyme powders. Proc. Natl. Acad. Sci. USA 1986, 83, 6810–6814. [Google Scholar] [CrossRef] [PubMed]
- Careri, G.; Milotti, E. Simulation of protonic fluctuations in hydrated protein powders. Phys. Rev. E 2003, 67, 051923. [Google Scholar] [CrossRef]
- Brovchenko, I.; Krukau, A.; Oleinikova, A.; Mazur, A.N. Water percolation governs polymorphic transitions and conductivity of DNA. Phys. Rev. Lett. 2006, 97, 137801. [Google Scholar] [CrossRef] [PubMed]
- Czerlinski, G.; Ypma, T. Domains of water molecules provide mechanisms of potentization in homeopathy. Water 2010, 2, 1–14. [Google Scholar]
- Czerlinski, G.; Ypma, T. The targets of information-carrying nanodomains. J. NanoSci. Nanotech. 2012, 12, 2239–2247. [Google Scholar] [CrossRef]
- Wang, G.M.; Sevick, E.M.; Mittag, E.; Searles, D.J.; Evans, D.J. Experimental demonstration of violations of the second law of thermodynamics for small systems and short time scales. Phys. Rev. Lett. 2002, 89, 050601. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.A. A greatly under-appreciated fundamental principle of physical organic chemistry. Intl. J. Mol. Sci. 2011, 12, 8316–8332. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, S.; Qiao, L. Surface tension of nanofluid-type fuels containing suspended nanomaterials. Nanoscale Res. Lett. 2012, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Debenedetti, P.G. Evaporation rate of water in hydrophobic confinement. Proc. Natl. Acad. Sci. 2012, 109, 4365–4370. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Suzuki, Y. Grand potential formalism of interfacial thermodynamics for critical nucleus. Nat. Sci. 2013, 5, 631–639. [Google Scholar] [CrossRef]
- Belloni, L.; Allweiss, L.; Guerrieri, F.; Pediconi, N.; Volz, T.; Pollicino, T.; Petersen, J.; Raimondo, G.; Dandri, M.; Levrero, M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J. Clin. Invest. 2012, 122, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Kumar, P.; Layer, R.; Willcox, S.; Gagan, J.R.; Griffith, J.D.; Dutta, A. Extrachromosomal microDNAs and chromosomal microdeletions in normal tissues. Science 2012, 336, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Detection of human papillomavirus L1 gene DNA fragments in postmortem blood and spleen after Gardasil® vaccination—A case report. Adv. Biosci. Biotechnol. 2012, 3, 1214–1224. [Google Scholar] [CrossRef]
- Rich, A.; Zhang, S. Z-DNA: The long road to biological function. Nature 2003, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Montagnier, L.; Aissa, J.; Ferris, S.; Montagnier, J.L.; Lavallee, C. Electromagnetic signals are produced by aqueous nanostructures derived from bacterial DNA sequences. Interdiscipl. Sci. Comp. Life Sci. 2009, 1, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Voeikov, V.L.; Naletov, V.I. Weak photon emission of non-linear chemical reactions of amino acids and sugars in aqueous solutions. In Biophotons; Chang, J.-J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 93–108. [Google Scholar]
- Montagnier, L.; Aissa, J.; Del Giudice, E.; Lavallee, C.; Tedeschi, A.; Vitiello, G. DNA waves and water. J. Phys. Conf. Ser. 2011, 306, 012007. [Google Scholar] [CrossRef]
- Voeikov, V.L.; Del Giudice, E. Water respiration—The basis of the living state. Water 2009, 1, 52–75. [Google Scholar]
- Preparata, G. QED Coherence and Matter; World Scientific: City, Singapore, 1995. [Google Scholar]
- Arani, R.; Bono, I.; Del Giudice, E.; Preparata, G. QED coherence and the thermodynamics of water. Int. J. Mod. Phys. B 1995, 9, 1813–1841. [Google Scholar] [CrossRef]
- Del Giudice, E.; Fuchs, E.; Vitiello, G. Collective molecular dynamics of a floating water bridge. Water 2010, 2, 69–82. [Google Scholar]
- Marchettini, N.; Del Giudice, E.; Voeikov, V.; Tiezzi, E. Water: A medium where dissipative structures are produced by a coherent dynamics. J. Theor. Biol. 2010, 265, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takeda, M.; Sato, T.; Yamazaki, Y.; Kaneko, K.; Ito, K.; Kato, H.; Inaba, H. In vivo imaging of spontaneous ultraweak photon emission from a rat’s brain correlated with cerebral energy metabolism and oxidative stress. Neurosci. Res. 1999, 34, 103–113. [Google Scholar] [CrossRef]
- Curtis, B.D.; Hurtak, J.J. Consciousness and quantum information processing: uncovering the foundation for a medicine of light. J. Altern. Complement. Med. 2004, 10, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Sim, S.B.; Kim, C.K.; Kim, J.; Choi, C.; You, H.; Soh, K.S. Spontaneous photon emission and delayed luminescence of two types of human lung cancer tissues: adenocarcinoma and squamous cell carcinoma. Cancer Lett. 2005, 229, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, C.; Lim, J.; You, H.; Sim, S.B.; Yom, Y.K.; Kim, E.H.; Soh, K.S. Measurements of spontaneous ultraweak photon emission and delayed luminescence from human cancer tissues. J. Altern. Complement. Med. 2005, 11, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Persinger, M.A. Developmental effects of perinatal exposure to extremely weak 7 Hz magnetic fields and nitric oxide modulation in the Wistar albino rat. Int. J. Dev. Neurosci. 2007, 25, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Fleischmann, M.; Preparata, G.; Talpo, G. On the “unreasonable” effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics 2002, 23, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Tafur, J.; Van Wijk, E.P.; Van Wijk, R.; Mills, P.J. Biophoton detection and low-intensity light therapy: A potential clinical partnership. Photomed. Laser Surg. 2010, 28, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-W. Quantum Coherent Water, Non-thermal EMF Effects, and Homeopathy. ISIS Report. Available online: http://www.i-sis.org.uk/Quantum_Coherent_Water_Homeopathy.php/ (accessed on 31 January 2013).
- Binhi, V.N.; Savin, A.V. Molecular gyroscopes and biological effects of weak extremely low-frequency. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002, 65, 051912. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Polcik, M.; Fukuma, T.; Sader, J.E.; Nakayama, Y.; Jarvis, S.P. Structured water layers adjacent to biological membranes. Biophys. J. 2006, 91, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. A few remarks on combined action of DC and AC magnetic fields on ion motion in a macromolecule. Bioelectromagnetics 2007, 28, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, L.; D’Emilia, E.; Grimaldi, S.; Lisi, A.; Bobkova, N.; Zhadin, M.N. Investigating the ICR effect in a Zhadin’s cell. Intl. J. Biomed. Sci. 2009, 5, 181–186. [Google Scholar]
- Zhadin, M. Quantum electrodynamical mechanisms of resonant effects development inside coherence domains at combined magnetic fields. In Proceedings of Action Progress in Electromagnetics, Research Symposium Abstracts, Moscow, Russia, 18–21 August 2009; p. 416.
- Liboff, A.R. The charge-to-mass ICR signature in weak ELF bioelectromagnetic effects. In Advances in Electromagnetic Fields in Living Systems, Chapter 6; Lin, J.C., Ed.; Springer Science+Business Media: New York, NY, USA, 2005; Volume 4. [Google Scholar]
- Vazquez, A. Optimal cytoplasmatic density and flux balance model under macromolecular crowding effects. J. Theor. Biol. 2010, 264, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, K.A.T.; Haymet, A.D.J.; Dill, K.A. A simple model of water and the hydrophobic effect. J. Am. Chem. Soc. 1998, 120, 3166–3175. [Google Scholar] [CrossRef]
- Latham, M.P.; Kay, L.E. Is buffer a good proxy for a crowded cell-like environment? A comparative NMR study of calmodulin side-chain dynamics in buffer and E. coli lysate. PloS One 2012, 7, e48226. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, L.; Grimaldi, S.; Lisi, A.; D’Emilia, E.; Bobkova, N.; Zhadin, M. Action of combined magnetic fields on aqueous solution of glutamic acid: the further development of investigations. Biomagn. Res. Technol. 2008, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhadin, M.; Giuliani, L. Some problems in modern bioelectromagnetics. Electromagn. Biol. Med. 2006, 25, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Liboff, A.R. The Ion Cyclotron Resonance Hypothesis. In Bioengineering and Biophysical Aspects of Electromagnetic Fields; Barnes, F., Greenebaum, B., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 261–292. [Google Scholar]
- Zhadin, M.N.; Novikov, V.V.; Barnes, F.S.; Pergola, N.F. Combined action of static and alternating magnetic fields on ionic current in aqueous glutamic acid solution. Bioelectromagnetics 1998, 19, 41–45. [Google Scholar] [CrossRef]
- Novikov, V.V.; Zhadin, M.N. Combined action of weak constant and variable low-frequency magnetic fields on ionic currents in aqueous solutions of amino acid. Biophysics 1994, 994, 41–45. [Google Scholar]
- Luby-Phelps, K. Cytoarchitecture and physical properties of cytoplasm: Volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000, 192, 189–221. [Google Scholar] [PubMed]
- Aggeli, A.; Nyrkova, I.A.; Bell, M.; Harding, R.; Carrick, L.; McLeish, T.C.; Semenov, A.N.; Boden, N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide beta-sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. USA 2001, 98, 11857–11862. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Waddington, L.; Easton, C.D.; Winkler, D.A.; Goodall, L.; Forsythe, J.; Hartley, P.G. Unzipping the role of chirality in nanoscale self-assembly of tripeptide hydrogels. Nanoscale 2012, 4, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Bruinsma, R.; Mason, T.G. Local chiral symmetry breaking in triatic liquid crystals. Nat. Comm. 2012, 3, 801. [Google Scholar] [CrossRef] [PubMed]
- Boncheva, M.; Andreev, S.A.; Mahadevan, L.; Winkleman, A.; Reichman, D.R.; Prentiss, M.G.; Whitesides, S.; Whitesides, G.M. Magnetic self-assembly of three-dimensional surfaces from planar sheets. Proc. Natl. Am. Sci. USA 2005, 102, 3924–3929. [Google Scholar] [CrossRef] [PubMed]
- Mirica, K.A.; Ilievski, F.; Ellerbee, A.K.; Shevkoplyas, S.S.; Whitesides, G.M. Using magnetic levitation for three dimensional self-assembly. Adv. Mater. 2011, 23, 4134–4140. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Butts, C.P.; Cheng, J.; Eastoe, J.; Russell, C.A.; Smith, G.N. Magnetic emulsions with responsive surfactants. Soft Matter 2012, 8, 7545–7546. [Google Scholar] [CrossRef]
- Brown, P.; Khan, A.M.; Armstrong, J.P.K.; Perriman, A.W.; Butts, C.P.; Eastoe, J. Magnetizing DNA and proteins using responsive surfactants. Adv. Matr. 2012, 24, 6244–6247. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Butts, C.P.; Eastoe, J.; Glatzel, S.; Grillo, I.; Hall, S.H.; Rogers, S.; Trickett, K. Microemulsions as tunable nanomagnets. Soft Matter 2012, 8, 11609–11612. [Google Scholar] [CrossRef]
- Banquy, X.; Kristiansen, K.; Lee, D.W.; Israelachvili, J.N. Adhesion and hemifusion of cytoplasmic myelin lipid membranes are highly dependent on the lipid composition. Biochim. Biophys. Acta. 2012, 1818, 402–410. [Google Scholar] [CrossRef]
- Sherman, I.A. Interfacial tension effects in the microvasculature. Microvasc. Res. 1981, 22, 296–307. [Google Scholar] [CrossRef]
- Rowlinson, J.S.; Widom, B. Molecular Theory of Capillarity; Dover Press: Mineola, NY, USA, 1982. [Google Scholar]
- Kashiwagi, T.; Kunishima, N.; Suzuki, C.; Tsuchiya, F.; Nikkuni, S.; Arata, Y.; Morikawa, K. The novel acidophilic structure of the killer toxin from halotolerant yeast demonstrates remarkable folding similarity with a fungal killer toxin. Structure 1997, 5, 81–94. [Google Scholar] [CrossRef]
- Mennerick, S.; Lamberta, M.; Shu, H.-J.; Hogins, J.; Wang, C.; Covey, D.F.; Eisenman, L.N.; Zorumski, C.F. Effects on membrane capacitance of steroids with antagonist properties at GABAA receptors. Biophys. J. 2008, 95, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Wu, K.; Zorumski, C.F.; Mennerick, S. Hydrophobic anions potently and uncompetitively antagonize GABA(A) receptor function in the absence of a conventional binding site. Br. J. Pharmacol. 2011, 164, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Chemical pathology of homocysteine. V. Thioretinamide, thioretinaco, and cystathionine synthase function in degenerative diseases. Ann. Clin. Lab. Sci. 2011, 41, 300–313. [Google Scholar]
- Shwartz, E.R.; Adamy, L. Effect of ascorbic acid on arylsulfatase activities and sulfated proteoglycan metabolism in chondrocyte cultures. J. Clin. Invest. 1977, 60, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Blank, R.D.; Doty, S.B. Vitamin C-sulfate inhibits mineralization in chondrocyte cultures: A caveat. Matrix Biol. 2001, 20, 99–106. [Google Scholar] [CrossRef]
- Verlangieri, J.; Mumma, R.O. In vivo sulfation of cholesterol by ascorbic acid 2-sulfate. Atherosclerosis 1973, 17, 37–48. [Google Scholar] [CrossRef]
- Chen, X.; Resh, M.D. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 49631–49637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-H.; Liu, L.-Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Individual ionic surface tension increments in aqueous solutions. Langmuir 2013, 29, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Paxson, A.T.; Dhiman, R.; Smith, J.D.; Varanasi, K.K. Enhanced condensation on lubricant-impregnated nanotextured surfaces. ACS Nano. 2012, 6, 10122–10129. [Google Scholar] [CrossRef] [PubMed]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. From superamphiphobic to amphiphilic polymeric surfaces with ordered hierarchical roughness fabricated with colloidal lithography and plasma nanotexturing. Langmuir 2011, 27, 3960–3969. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.; Kataky, R. Electron transport in supported and tethered lipid bilayers modified with bioelectroactive molecules. J. Phys. Chem. B 2012, 116, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A. Nanoscale thermodynamics of biological interfacial tension. Proc. R. Soc. A 2011, 467, 559–568. [Google Scholar] [CrossRef]
- Fernandez, A. Epistructural tension promotes protein associations. Phys. Rev. Lett. 2012, 108, 188102. [Google Scholar] [CrossRef] [PubMed]
- Brecher, G.; Bessis, M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: A critical review. Blood 1972, 40, 333–344. [Google Scholar] [PubMed]
- Absolom, D.R. Measurement of surface properties of phagocytes, bacteria, and other particles. Methods Enzymol. 1986, 132, 16–95. [Google Scholar] [PubMed]
- Gallez, D.; Coakley, W.T. Interfacial instability at cell membranes. Prog. Biophys. Mol. Biol. 1986, 48, 155–199. [Google Scholar] [CrossRef]
- Berthelot, M. Sur quelques phénomènes de dilatation forcée des liquids. Annales. de Chimie. et de Physique. 1850, 30, 232–237, (English translation). [Google Scholar]
- Larios, E.; Gruebele, M. Protein stability at negative pressure. Methods 2010, 52, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Grigera, J.R.; Andres, N.; McCarthy, A.N. The behavior of the hydrophobic effect under pressure and protein denaturation. Biophys. J. 2010, 98, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Defay, R.; Prigogine, I. Surface Tension and Adsorption; Wiley: Hoboken, NJ, USA, 1966. [Google Scholar]
- Janmey, P.A. Gel-sol transition of the cytoplasm and its regulation. AIP Conf. Proc. 1980, 226, 304–325. [Google Scholar]
- Debenedetti, P.G.; Stillinger, F.H. Supercooled liquids and the glass transition. Nature 2001, 410, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Torre, R.; Bartolini, P.; Righini, R. Structural relaxation in supercooled water by time-resolved spectroscopy. Nature 2004, 428, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Barstow, B.; Tate, M.W.; Gruner, S.M. Evidence for liquid water during the high-density to low-density amorphous ice transition. Proc. Natl. Am. Sci. USA 2009, 106, 4596–4600. [Google Scholar] [CrossRef] [PubMed]
- Pelling, A.E.; Dawson, D.W.; Carreon, D.M.; Christiansen, J.J.; Shen, R.R.; Teitell, M.A.; Gimzewski, J.K. Distinct contributions of microtubule subtypes to cell membrane shape and stability. Nanomedicine 2007, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.H.; Trepat, X.; Park, C.Y.; Lenormand, G.; Oliver, M.N.; Mijailovich, S.M.; Hardin, C.; Weitz, C.A.; Butler, J.P.; Fredberg, J.J. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl. Am. Sci. USA 2009, 106, 10632–10637. [Google Scholar]
- Huang, D.M.; Chandler, D. The hydrophobic effect and the influence of solute-solvent attractions. J. Phys. Chem. B 2002, 106, 2047–2053. [Google Scholar] [CrossRef]
- Woods, A.; Couchman, J.R.; Höök, M. Heparan sulfate proteoglycans of rat embryo fibroblasts. A hydrophobic form may link cytoskeleton and matrix components. J. Biol. Chem. 1985, 260, 10872–10879. [Google Scholar] [PubMed]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers. Dis. 2011, 276393. [Google Scholar] [CrossRef] [PubMed]
- Szent-Györgyi, A.G. Calcium regulation of muscle contraction. Biophys. J. 1975, 15, 707–723. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Hama, Y.; Sasaki, T.; Kubodera, A. Elevated uptake of 67Ga and increased heparan sulfate content in liver-damaged rats. Eur. J. Nucl. Med. 1983, 8, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Ando, I.; Hiraki, T.; Hisada, K. 67Ga-binding substances in stomach, small intestine, pancreas, and muscle. Eur. J. Nucl. Med. 1985, 11, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Sasaki, T.; Kojima, S.; Kubodera, A. 67Ga accumulation and heparan sulfate metabolism in lysosomes. Eur. J. Nucl. Med. 1984, 9, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Thrombohemorrhagic Phenomena; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1966. [Google Scholar]
- Selye, H. In Vivo: The Case for Supramolecular Biology; Liveright Publishing Corporation: New York, NY, USA, 1967. [Google Scholar]
- Mastruserio, D.N.; Nguyen, E.Q.; Nielsen, T.; Hessel, A.; Pellegrini, A.E. Calciphylaxis associated with metastatic breast carcinoma. J. Am. Acad. Dermatol. 1999, 41, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.K.; Bellovich, K.; McCullough, P.A. Treatment of severe metastatic calcification and calciphylaxis in dialysis patients. Int. J. Nephrol. 2011, 2011. Article ID 701603. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Friedman, J.M. Charge density-dependent modifications of hydration shell waters by Hofmeister ions. J. Am. Chem. Soc. 2009, 131, 11010–11018. [Google Scholar] [CrossRef] [PubMed]
- Tielrooij, K.J.; Garcia-Araez, N.; Bonn, M.; Bakker, H.J. Cooperativity in ion hydration. Science 2010, 328, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Volumetric properties of molten salt hydrates. J. Chem. Eng. Data 2013, 58, 488–491. [Google Scholar] [CrossRef]
- Rezwan, K.; Meier, L.P.; Rezwan, M.; Vöörös, J.; Textor, M.; Gauckler, L.J. Bovine serum albumin adsorption onto colloidal Al2O3 particles: a new model based on zeta potential and UV-vis measurements. Langmuir 2004, 20, 10055–10061. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Somasundaran, P. Reversal of bubble charge in multivalent inorganic salt solutions: Effect of aluminum. J. Colloid. Interface Sci. 1992, 148, 587–591. [Google Scholar] [CrossRef]
- Nday, C.; Salifoglou, A. The influence of the environmental metallotoxin Al(III) on neuronal cell structures linked to neurodegeneration. J. Agroaliment. Proc. Tech. 2012, 18, 208–211. [Google Scholar]
- Lipinski, B.; Jeljaszewicz, J. A hypothesis for the pathogenesis of the generalized Shwartzman reaction. J. Infect. Dis. 1969, 120, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Riddick, T. Control of Colloid Stability through Zeta Potential (with a Closing Chapter on its Relationship to Cardiovascular Disease); Livingston Pub. Co.: Wynnewood, PA, USA, 1968. [Google Scholar]
- Armenante, P.M. Coagulation & Flocculation. Available online: http://cpe.njit.edu/dlnotes/che685/cls07–1.pdf (accessed on 23 January 2013).
- Droste, R.L. Theory and Practice of Water and Wastewater Treatment; John Wiley & Sons: New York, NY, USA, 1997; pp. 384–415. [Google Scholar]
- Del Giudice, E.; Doglia, S.; Milani, M.; Smith, C.W.; Vitiello, G. Magnetic flux quantization and Josephson behaviour in living systems. Phys. Scr. 1989, 40, 786. [Google Scholar] [CrossRef]
- Hunt, R.W.; Zavalin, A.; Bhatnagar, A.; Chinnasamy, S.; Das, K.C. Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Int. J. Mol. Sci. 2009, 10, 4515–4558. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.I.; Jones, B.M.; Bhattacharjee, S.; Percy, M.E.; Zhao, Y.; Lukiw, W.J. Metal-sulfate induced generation of ROS in human brain cells: Detection using an isomeric mixture of 5- and 6-carboxy-2,7-dichlorofluoresce in diacetate (carboxy-DCFDA) as a cell permeant tracer. Int. J. Mol. Sci. 2012, 13, 9615–9626. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.; Baisakhi, B.; Mohapatro, M.K.; Panda, B.B. Aluminium triggers genotoxic adaptation to methyl mercuric chloride and ethyl methane sulfonate, but not to maleic hydrazide in plant cells in vivo. Mutat. Res. 2000, 465, 1–9. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Panda, B.B. Aluminium-induced DNA damage and adaptive response to genotoxic stress in plant cells are mediated through reactive oxygen intermediates. Mutagenesis 2010, 25, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Achary, V.M.; Parinandi, N.L.; Panda, B.B. Aluminum induces oxidative burst, cell wall NADH peroxidase activity, and DNA damage in root cells of Allium cepa L. Enviro. Mol. Mutagen. 2012, 53, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Baylor, N.W.; Egan, W.; Richman, P. Aluminum salts in vaccines–US perspective. Vaccine 2002, 20, S18–S23. [Google Scholar] [CrossRef]
- Exley, C. The pro-oxidant activity of aluminum. Free Radic. Biol. Med. 2004, 36, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Mujika, J.I.; Ruiperez, F.; Infante, I.; Ugalde, J.M.; Exley, C.; Lopez, X. Pro-oxidant activity of aluminum: stabilization of the aluminum superoxide radical ion. J. Phys. Chem. A 2011, 115, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. The coordination chemistry of aluminium in neurodegenerative disease. Coord. Chem. Rev. 2012, 256, 2142–2146. [Google Scholar] [CrossRef]
- Pogue, A.I.; Li, Y.Y.; Cui, J.-G.; Zhao, Y.; Kruck, T.P.A.; Percy, M.E.; Tarr, M.A.; Lukiw, W.J. Characterization of an NF-jB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J. Inorg. Biochem. 2009, 103, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Lupidi, G.; Angeletti, M.; Eleuteri, A.M.; Fioretti, E.; Marini, S.; Gioia, M.; Coletta, M. Aluminum modulation of proteolytic activities. Coord. Chem. Rev. 2002, 228, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Womack, F.C.; Colowick, S.P. Proton-dependent inhibition of yeast and brain hexokinases by aluminum in ATP preparations. Proc. Natl. Acad. Sci. USA 1979, 76, 5080–5084. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Blass, J.P. Inhibition of brain glycolysis by aluminum. J. Neurochem. 1984, 42, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Price, N.C.; Birchall, J.D. Aluminum inhibition of hexokinase activity in vitro: A study in biological availability. J. Inorg. Biochem. 1994, 54, 297–304. [Google Scholar] [CrossRef]
- Li, L. The biochemistry and physiology of metallic fluoride: Action, mechanism, and implications. Crit. Rev. Oral Biol. Med. 2003, 4, 100–114. [Google Scholar] [CrossRef]
- Isaacson, R.A.; Varner, J.A.; Jensen, K.F. Toxin-induced blood vessel inclusions caused by the chronic administration of aluminum and sodium fluoride and their implications for dementia. Ann. NY Acad. Sci. 1997, 825, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Varner, J.A.; Jensen, K.F.; Horvath, W.; Isaacson, R.L. Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: Alterations in neuronal and cerebrovascular integrity. Brain Res. 1998, 784, 284–298. [Google Scholar] [CrossRef]
- Kaur, T.; Bijarnia, R.K.; Nehru, B. Effect of concurrent chronic exposure of fluoride and aluminum on rat brain. Drug Chem. Toxicol. 2009, 32, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wittinghofer, A. Signaling mechanistics: Aluminum fluoride for molecule of the year. Curr. Biol. 1997, 7, R682–R685. [Google Scholar] [CrossRef]
- Braig, K.; Menz, R.I.; Montgomery, M.G.; Leslie, A.G.W.; Walker, J.E. Structure of bovine mitochondrial F1-ATPase inhibited by Mg2+ADP and aluminium fluoride. Structure 2000, 8, 567–573. [Google Scholar] [CrossRef]
- Miles, R.D.; Gorrell, A.; Ferry, J.G. Evidence for a transition state analog, MgADP-aluminum fluoride-acetate, in acetate kinase from Methanosarcina thermophila. J. Biol. Chem. 2002, 277, 22547–22552. [Google Scholar] [CrossRef] [PubMed]
- Maruta, S.; Henry, G.D.; Sykes, B.D.; Ikebe, M. Formation of the stable myosin-ADP-aluminum fluoride and myosin-ADP-beryllium fluoride complexes and their analysis using 19F NMR. J. Biol. Chem. 1993, 268, 7093–7100. [Google Scholar] [PubMed]
- Werber, M.M.; Peyser, Y.M.; Muhlrad, A. Characterization of stable beryllium fluoride, aluminum fluoride, and vanadate containing myosin subfragment 1-nucleotide complexes. Biochemistry 1992, 31, 7190–7197. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, M.A.; Timofeev, V.P.; Levitsky, D.I. The difference between ADP-beryllium fluoride and ADP-aluminum fluoride complexes of the spin-labeled myosin subfragment 1. FEBS Lett. 1995, 371, 261–263. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Lee, Y.J.; Hsu, G.S. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci. 2012, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Haley, B. Mercury toxicity: Genetic susceptibility and synergistic effects. Medical Veritas. 2005, 2, 535–542. [Google Scholar] [CrossRef]
- Kong, S.; Liochev, S.; Fridovich, I. Aluminum (III) facilitates the oxidation of NADH by the superoxide anion. Free Radic. Biol. Med. 1992, 13, 79–81. [Google Scholar] [CrossRef]
- Vota, D.M.; Crisp, R.L.; Nesse, A.B.; Vittori, D.C. Oxidative stress due to aluminum exposure induces eryptosis which is prevented by erythropoietin. J. Cell. Biochem. 2012, 113, 1581–1589. [Google Scholar] [PubMed]
- Burrell, S.-A.M.; Exley, C. There is (still) too much aluminium in infant formulas. BMC Pediatr. 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.R.; Bohrer, D.; Garcia, S.C.; do Nascimento, P.C.; Noremberg, S. Aluminum content in intravenous solutions for administration to neonates: Role of product preparation and administration methods. JPEN J. Parenter. Enteral. Nutr. 2010, 34, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Vittori, D.; Nesse, A.; Pérez, G.; Garbossa, G. Morphologic and functional alterations of erythroid cells induced by long-term ingestion of aluminium. J. Inorg. Biochem. 1999, 76, 113–120. [Google Scholar] [CrossRef]
- Vittori, D.; Garbossa, G.; Lafourcade, C.; Pérez, G.; Nesse, A. Human erythroid cells are affected by aluminium. Alteration of membrane band 3 protein. Biochim. Biophys. Acta 2002, 1558, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Lauritzen, A.; Davidson, R.; Lentz-Marino, L. Is endothelial nitric oxide synthase a moonlighting protein whose day job is cholesterol sulfate synthesis? Implications for cholesterol transport, diabetes and cardiovascular disease. Entropy 2012, 14, 2492–2530. [Google Scholar] [CrossRef] [Green Version]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.; Jablonska-Gajewicz, J.; Kordek, R. Hydrogen bonds of interfacial water in human breast cancer tissue compared to lipid and DNA interfaces. JBC 2011, 2, 158–169. [Google Scholar] [CrossRef]
- Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; Schoenenberger, C.-A. The nanomechanical signature of breast cancer. Nat. Nano 2012, 7, 757–765. [Google Scholar]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS One 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nano 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kai, K.; Choi, D.S.; Iwamoto, T.; Nguyen, Y.H.; Wong, H.; Landis, M.D.; Ueno, N.T.; Chang, J.; Qin, L. Microfluidics separation reveals stem-cell-like deformability of tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2012, 109, 18707–18712. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Mythreye, K.; O'Brien, E.T.; Berchuck, A.; Blobe, G.C.; Superfine, R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Pike, S.; Gassmann, W.; Baskin, T.I. Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: Evidence that these responses are mediated by a glutamate receptor. Plant. Cell. Physiol. 2003, 44, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, B.R.; Beall, P.T.; Wible, L.J.; Mace, M.L.; Turner, D.S.; Cailleau, R.M. Variations in cell form and cytoskeleton in human breast carcinoma cells in vitro ,in vitro. Cancer Res. 1980, 40, 3118–3129. [Google Scholar] [PubMed]
- Schedin, P.; Keely, P.J. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 2011, 3, a003228. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Vesentini, S.; Radaelli, A.; Buehler, M.J. Hierarchical structure and nanomechanics of collagenmMicrofibrils from the atomistic scale up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Brown, R.A.; Mudera, V. Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J. Biomed. Mat. Res. Part A 2007, 83A, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.V.; Soto, A.M.; Calabro, J.M.; Ucci, A.A.; Sonnenschein, C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 2004, 117, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.V.; Calabro, J.M.; Soto, A.M.; Sonnenschein, C. Stromal Regulation of Neoplastic Development: Age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 2005, 167, 1405–1410. [Google Scholar] [CrossRef]
- Booth, B.W.; Boulanger, C.A.; Anderson, L.H.; Smith, G.H. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene 2011, 30, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, C.; Soto, A.M. Cancer genes: The vestigial remains of a fallen theory. In Genetic Explanations: Sense and Nonsense; Krimsky, S., Gruber, J., Eds.; Harvard University Press: Cambridge, MA, USA, 2013; pp. 81–93. [Google Scholar]
- Sonnenschein, C.; Soto, A.M. The Society of Cells: Cancer and Control of Cell Proliferation; Bios Scientific Publishers: Oxford, UK, 1999. [Google Scholar]
- Sonnenschein, C.; Soto, A.M. Theories of carcinogenesis: An emerging perspective. Seminars in Cancer Biology 2008, 18, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J. Appl. Toxicol. 2006, 26, 191–197. [Google Scholar] [CrossRef]
- Silva, N.; Peiris-John, R.; Wickremasinghe, R.; Senanayake, H.; Sathiakumar, N. Cadmium a metalloestrogen: Are we convinced? JAT 2012, 32, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Charles, L.M.; Barr, L.; Martin, C.; Polwart, A.; Darbre, P.D. Aluminium in human breast tissue. J. Inorg. Biochem. 2007, 101, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Sappino, A.P.; Buser, R.; Lesne, L.; Gimelli, S.; Bena, F.; Belin, D.; Mandriota, S.J. Aluminium chloride promotes anchorage-independent growth in human mammary epithelial cells. JAT 2012, 32, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Al Zubaidy, E.A.H.; Mohammad, F.S.; Bassioni, G. Effect of pH, salinity and temperature on aluminum cookware. Int. J. Electrochem. Sci. 2011, 6, 6424–6441. [Google Scholar]
- Tomljenovic, L.; Shaw, C.A. Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? J. Inorg. Biochem. 2011, 105, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Lauritzen, A.; Davidson, R.M.; Lentz-Marino, L. Is encephalopathy a mechanism to renew sulfate in autism? Entropy 2013, 15, 372–406. [Google Scholar] [CrossRef] [Green Version]
- McGrath, K.G. An earlier age of breast cancer diagnosis related to more frequent use of antiperspirants/deodorants and underarm shaving. Eur. J. Cancer Prev. 2003, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kanthou, C.; Tozer, G.M. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood 2002, 99, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Yokoyama, K.; Mihashi, K.; Kodama, T.; Suzuki, M. Hyper-mobile water is induced around actin filaments. Biophys. J. 2003, 85, 3154–3161. [Google Scholar] [CrossRef]
- Wazawa, T.; Sagawa, T.; Ogawa, T.; Morimoto, N.; Kodama, T.; Suzuki, M. Hypermobility of water around actin filaments revealed using pulse-field gradient spin-echo 1H NMR and fluorescence spectroscopy. Biochem. Biophys. Res. Commun. 2011, 404, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.M.; Narayana, P.A. DTI parameter optimization at 3.0 T: Potential application in entire normal human brain mapping and multiple sclerosis research. Medicamundi 2005, 491, 30–45. [Google Scholar]
- Basser, P.J. Relationships between diffusion tensor and q-space MRI. Mag. Res. Med. 2002, 47, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 4350–455. [Google Scholar] [CrossRef] [PubMed]
- Horsfield, M.A.; Jones, D.K. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases—A review. NMR Biomed. 2002, 15, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.; Miller, J.; Mukherjee, P.; Hüppi, P.S. Diffusion tensor imaging of normal and injured developing human brain—A technical review. NMR Biomed. 2002, 15, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mori, S. Introduction to Diffusion Tensor Imaging; Elsevier Science: Maryland Heights, MO, USA, 2007. [Google Scholar]
- Barnea-Goraly, N.; Kwon, H.; Menon, V.; Eliez, S.; Lotspeich, L.; Reiss, A.L. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psych. 2004, 55, 323–326. [Google Scholar] [CrossRef]
- Brito, A.R.; Vasconcelos, M.M.; Domingues, R.C.; Hygino da Cruz, L.C., Jr.; Rodrigues Lde, S.; Gasparetto, E.L.; Calcada, C.A. Diffusion tensor imaging findings in school-aged autistic children. J. Neuroimag. 2009, 19, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Van Zijl, P. From the diffusion coefficient to the diffusion tensor. NMR Biomed. 2002, 15, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, M.S.; Friedman, J.; Buchsbaum, B.R.; Chu, K.W.; Hazlett, E.A.; Newmark, R.; Schneiderman, J.S.; Torosjan, Y.; Tang, C.; Hof, P.R.; et al. Diffusion tensor imaging in schizophrenia. Biol. Psch. 2006, 60, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Higano, S.; Tamura, H.; Mugikura, S.; Takahashi, S. Colour-coded fractional anisotropy images: differential visualisation of white-matter tracts–preliminary experience. Neuroradiology 2002, 44, 822–824. [Google Scholar] [PubMed]
- Friese, U.; Meindl, T.; Herpertz, S.C.; Reiser, M.F.; Hampel, H.; Teipel, S.J. Diagnostic utility of novel MRI-based biomarkers for Alzheimer’s disease. JAD 2010, 20, 477–490. [Google Scholar] [PubMed]
- Inglese, M.; Bester, M. Diffusion imaging in multiple sclerosis: Research and clinical implications. NMR Biomed. 2010, 23, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Absinta, M.; Sormani, M.P.; Ghezzi, A.; Bertolotto, A.; Montanari, E.; Comi, G.; Filippi, M. In vivo assessment of cervical cord damage in MS patients: A longitudinal diffusion tensor MRI study. Brain 2007, 130, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Cercignani, M.; Bozzali, M.; Iannucci, G.; Comi, G.; Filippi, M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis. J. Neurol. 2002, 249, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Saindane, A.M.; Ge, Y.; Babb, J.S.; Johnson, G.; Mannon, L.J.; Herbert, J.; Grossman, R.I. Microvascular abnormality in relapsing-remitting multiple sclerosis: Perfusion MR imaging findings in normal-appearing white matter. Radiology 2004, 231, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.M.; Walimuni, I.S.; Abid, H.; Wolinsky, J.S.; Narayana, P.A. Multimodal quantitative MRI investigation of brain tissue neurodegeneration. JMRI 2012, 35, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Schmerr, M.J.; Jenny, A.; Cutlip, R.C. Use of capillary sodium dodecyl sulfate gel electrophoresis to detect the prion. J. Chromatogr. B. 1997, 697, 223–229. [Google Scholar] [CrossRef]
- Kretlow, A.; Wang, Q.; Kneipp, J.; Lasch, P.; Beekes, M.; Miller, L.; Naumann, D. FTIR-microspectroscopy of prion-infected nervous tissue. Biochim. Biophys. Acta 2006, 1758, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Cerpa, R.; Cohen, F.E.; Kuntz, I.D. Conformational switching in designed peptides: The helix/sheet transition. Folding Design 1996, 1, 91–101. [Google Scholar] [CrossRef]
- Fernández, A.; Scotty, R. Dehydron: A structurally encoded signal for protein interaction. Biophys. J. 2003, 85, 1914–1928. [Google Scholar] [CrossRef]
- Fernández, A. Insufficient hydrogen-bond desolvation and prion-related disease. Eur. J. Biochem. 2002, 269, 4165–4168. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, F.; Etinger-Tulczynska, R. Beitrag zur wirkungsweise der phagozytoseerre- genden immunkorpcr (in German). Zbl. Bakt. Abt. I 1929, 114, 252. [Google Scholar]
- Jandl, J.H.; Simmons, R.L. The agglutination and sensitization of red cells by metallic cations: Interactions between multivalent metals and the red-cell membrane. Brit. J. Haemat. 1957, 3, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.; Cowan, K.M.; Osler, A.G.; Mayer, M.M. Studies on the role of Ca2+ and Mg2+ in complement fixation and immune hemolysis. J. Immunol. 1953, 71, 359–367. [Google Scholar] [PubMed]
- Hinz, C.F., Jr.; Pillemer, L. The requirement for the properdin system in the hemolysis of human erythrocytes treated with tannic acid. J. Clin. Invest. 1955, 34, 912. [Google Scholar]
- Chernomordik, L.V.; Kozlov, M.M. Protein-lipid interplay in fusion and fission of biological membranes. Ann. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; McMahon, H.T. Mechanisms of membrane fusion: Disparate players and common principles. Nature Rev. Mol. Cell. Biol. 2008, 9, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Lee, C.T.; Chmelka, B.F.; Israelachvili, J.N. General hydrophobic interaction potential for surfactant/lipid bilayers from direct force measurements between light-modulated bilayers. Proc. Natl. Acad. Sci. USA 2011, 108, 15699–15704. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.M.; Sarkar, M. Membrane fusion induced by small molecules and ions. J. Lipids 2011, 2011. Article ID 528784. [Google Scholar]
- Haque, M.E.; Koppaka, V.; Axelsen, P.H.; Lentz, B.R. Properties and structures of the influenza and HIV fusion peptides on lipid membranes: Implications for a role in fusion. Biophys. J. 2005, 89, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Spear, P.G. Herpes viruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 2001, 103, 503–510. [Google Scholar] [CrossRef]
- Liboff, A.R. Electromagnetic vaccination. Med. Hypotheses 2012, 79, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Twenhafel, N.A.; Mattix, M.E.; Johnson, J.C.; Robinson, C.G.; Pratt, W.D.; Cashman, K.A.; Wahl-Jensen, V.; Terry, C.; Olinger, G.G.; Hensley, L.E.; et al. Pathology of experimental aerosol Zaire ebolavirus infection in rhesus macaques. Vet. Pathol. 2012, in press. [Google Scholar] [CrossRef] [PubMed]
- Salari, V.; Tuszynski, J.; Rahnama, M.; Bernroider, G. Plausibility of quantum coherent states in biological systems. J. Phys.Conf. Ser. 2011, 306, 012075. [Google Scholar] [CrossRef]
- Sear, R.P. The cytoplasm of living cells: A functional mixture of thousands of components. J. Phys. 2005, 17, S3587. [Google Scholar] [CrossRef]
- Hazlewood, C.F. A role for water in the exclusion of cellular sodium—Is a sodium pump needed? Cardiovasc. Dis. Bull. Texas Heart Inst. 1975, 2, 83–104. [Google Scholar]
- Leterrier, J.F. Water and the cytoskeleton. Cell. Mol. Biol. (Noisy-le-grand) 2001, 47, 901–923. [Google Scholar]
- McIntyre, G.I. Increased cell hydration promotes both tumor growth and metastasis: A biochemical mechanism consistent with genetic signatures. Med. Hypotheses 2007, 69, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Toral, C.; Mendoza-Garrido, M.E.; Azorín, E.; Hernández-Gallegos, E.; Gomora, J.C.; Delgadillo, D.M.; Solano-Agama, C.; Camacho, J. Effect of extracellular matrix on adhesion, viability, actin cytoskeleton and K+ currents of cells expressing human ether à go-go channels. Life Sci. 2007, 81, 255–265. [Google Scholar] [CrossRef]
- Ling, G.N. A Physical Theory of the Living State: The Association-Induction Hypothesis; Blaisdell Publishing Company: New York, NY, USA, 1962. [Google Scholar]
- Jaeken, L.; Matveev, V.V. Coherent behavior and the bound state of water and K+ imply another model of bioenergetics: Negative entropy instead of high-energy bonds. The Open Biochem. J. 2012, 6, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Gryder, B.E.; Nelson, C.W.; Shepard, S.S. Biosemiotic entropy of the genome: Mutations and epigenetic imbalances resulting in cancer. Entropy 2013, 15, 234–261. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C. The somatic mutation theory of cancer: Growing problems with the paradigm? BioEssays 2004, 26, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.S.; Yazzie, B.; Middaugh, C.R. Polyanions and the proteome. Mol. Cell. Proteomics 2004, 3, 746–769. [Google Scholar] [CrossRef] [PubMed]
- Zahl, P.-H.; Mæhlen, J.; Welch, G. The natural history of invasive breast cancers detected by screening mammography. Arch. Intern. Med. 2008, 168, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Davidson, R.M.; Lauritzen, A.; Seneff, S. Biological Water Dynamics and Entropy: A Biophysical Origin of Cancer and Other Diseases. Entropy 2013, 15, 3822-3876. https://doi.org/10.3390/e15093822
Davidson RM, Lauritzen A, Seneff S. Biological Water Dynamics and Entropy: A Biophysical Origin of Cancer and Other Diseases. Entropy. 2013; 15(9):3822-3876. https://doi.org/10.3390/e15093822
Chicago/Turabian StyleDavidson, Robert M., Ann Lauritzen, and Stephanie Seneff. 2013. "Biological Water Dynamics and Entropy: A Biophysical Origin of Cancer and Other Diseases" Entropy 15, no. 9: 3822-3876. https://doi.org/10.3390/e15093822
APA StyleDavidson, R. M., Lauritzen, A., & Seneff, S. (2013). Biological Water Dynamics and Entropy: A Biophysical Origin of Cancer and Other Diseases. Entropy, 15(9), 3822-3876. https://doi.org/10.3390/e15093822




