Thermoelectric Effects under Adiabatic Conditions
Abstract
:1. Introduction
- The experimental procedures and the collected anomalous data is reviewed.
- The well-known explanation for thermoelectric effects (Peltier, Seebeck and Thomson) is summarized as a manifestation of conventional adiabatic effects.
- Built-in properties of semiconductor are discussed.
- Adiabatic effects in a gas phase (as in the atmosphere) are described as analogous to effects due to charge carriers in semiconductors.
- The Diffusion-Diffusion Model is presented and compared to the well-known Diffusion and Drift Model.
- Thermoelectric materials are discussed as special kind of semiconductors capable of supporting observable diffusion-diffusion model phenomena.
- Two hypothesized new thermoelectric effects are presented.
- The experiments are discussed in light of the hypothesized effects.
- A simulation is made available to the reader for desk-top experimentation of adiabatic phenomena.
2. Experiments
2.1. Experiments 1
- Poor thermal contacts between the thermocouple and the sample surface,
- Lack of steady state during the measurement of the temperature and voltage, and lack of simultaneity between temperature and voltage measurements.
- Non-linearity in the voltage-temperature response.
- Lack of co-location in measurements of voltage and temperature.
- Chemical reaction between the sample and the thermocouple materials, including spurious Seebeck effects between the voltage probe and the thermoelectric sample.
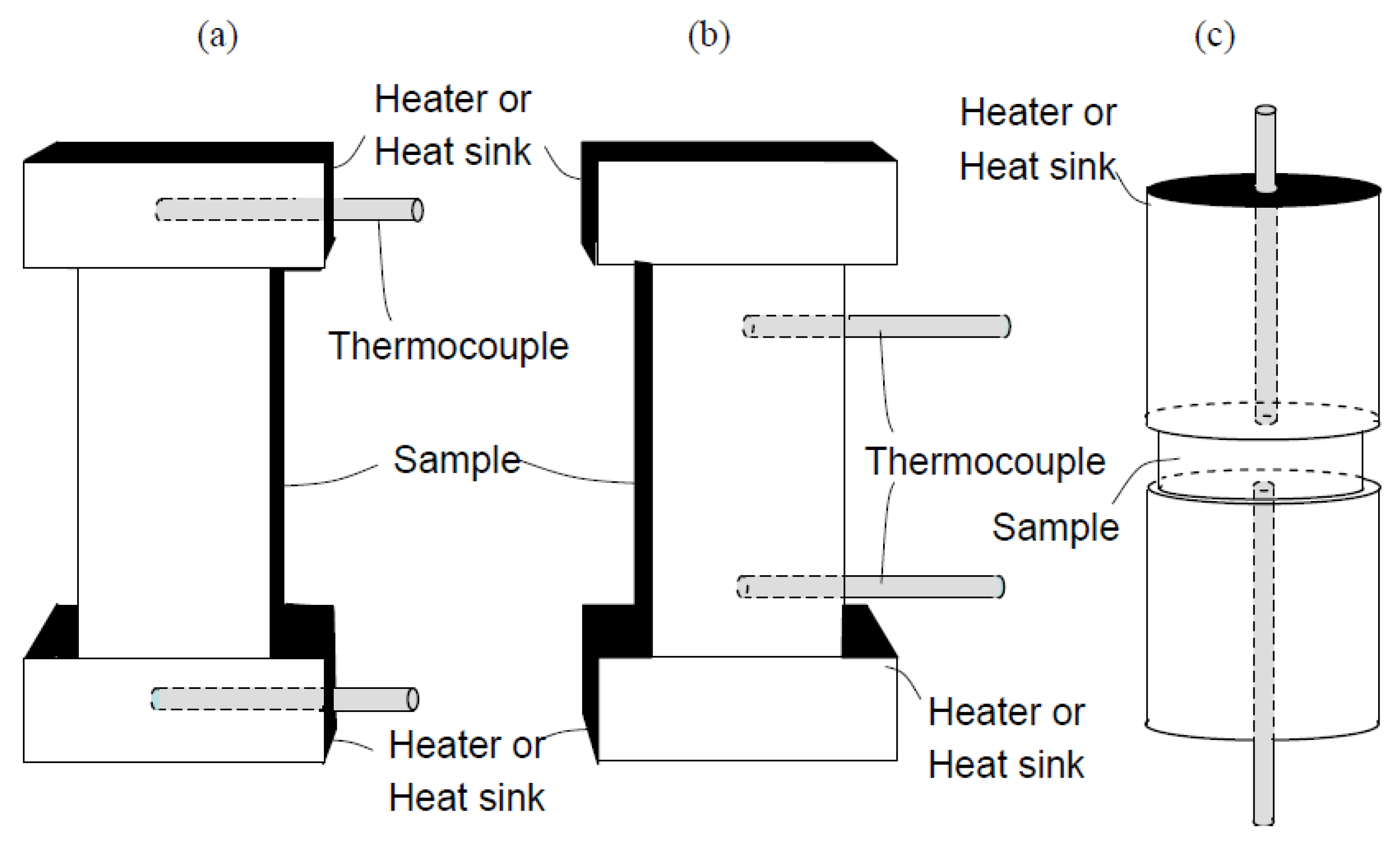
- The uniaxial 4-point geometry of their apparatus allowed the thermocouples to make direct contact with the sample and produced an isothermal temperature distribution on the surface of the sample.
- Compressive springs were used to exert a uniform force onto the sample allowing good thermo-mechanical contacts.
- The spurious Seebeck effects caused by a temperature difference between the voltage probe and the sample were eliminated by using near-zero Seebeck coefficient materials with high thermal conductivity (tungsten and niobium) in the probe in combination with a high Seebeck material in the thermocouple. Lack of co-location in measurements of voltage and temperature.
- Chemical reactions were eliminated by placing polycrystalline graphite foil between the sample and the thermocouple.
- An algorithmic approach (delta method) was used to account for the lack of perfect thermal steady state, and correct the thermal drift in the sample.

- “During high temperature measurements, there is often a noticeable voltage offset, which can range from a few µV due to the electronics to almost 1mV, which can sometimes be observed at high temperatures. The origin of the large offset and its effect on the accuracy of the measured Seebeck coefficient is unknown.”
- “we and many other thermoelectric groups have observed a temperature offset at zero voltage difference in an attempt to measure Seebeck voltage across thermoelectric samples…. This is commonly observed and subtracted in Seebeck measurement systems including those sold commercially. An offset of about 0.1 K is clearly visible in the paper we published on the measurement of Seebeck coefficient in 2011. This temperature offset can vary greatly and depends on temperature and the type of material tested, and not simply due to errors in thermometry. I have had no less than three students and postdocs study this effect and have collected much data that characterizes it but none have been able to explain this effect.”
2.2. Experiment 2
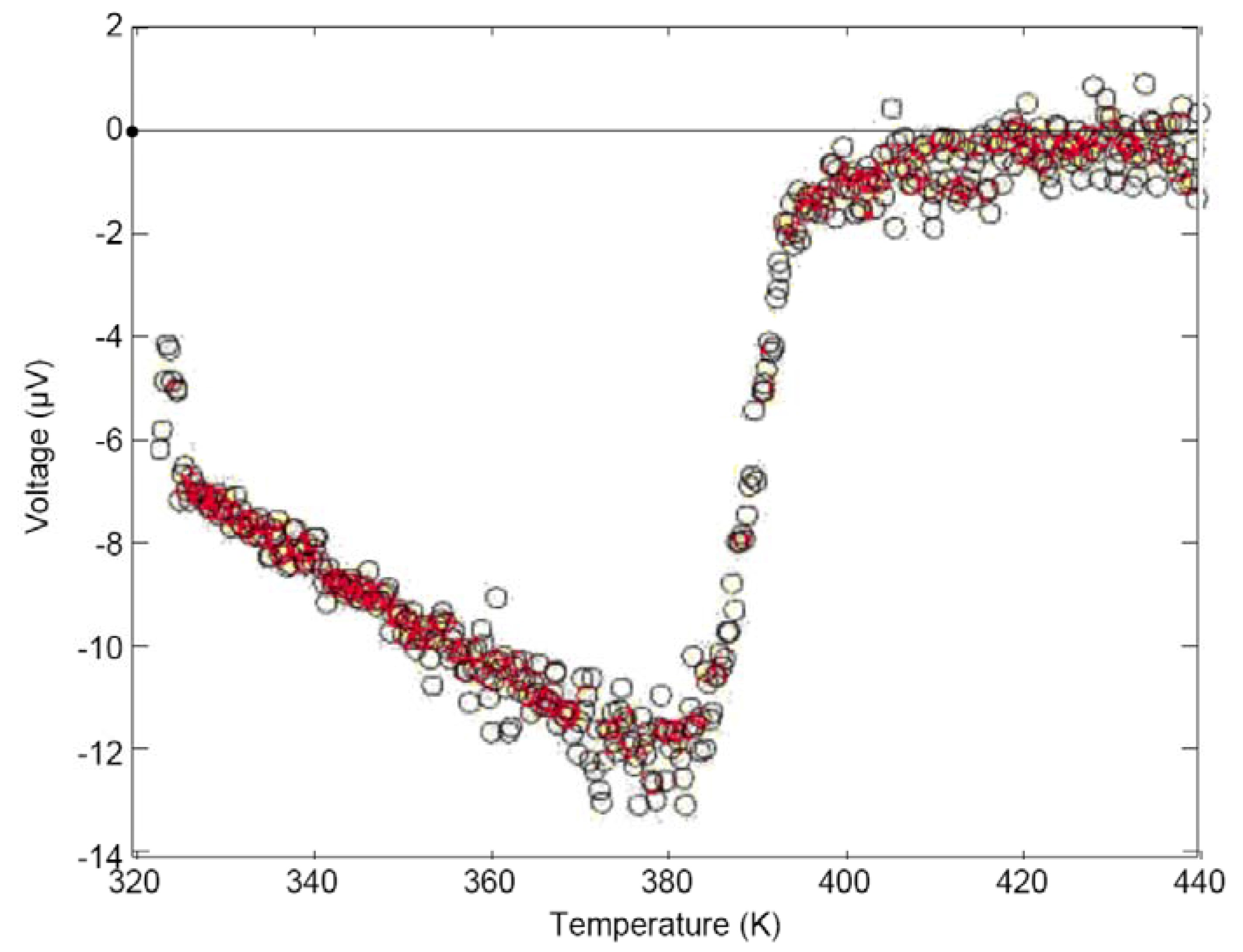
- “We have no conventional explanation for this effect. From the extent of the data it is clearly reproducible and a property of the material tested because of the known transition in the material at 390K.”
3. Theoretical Explanation
3.1. Known Adiabatic Phenomena in Semiconductors
3.2. Build-in Properties across Junctions

3.3. Adiabatic Phenomena–An Analogy
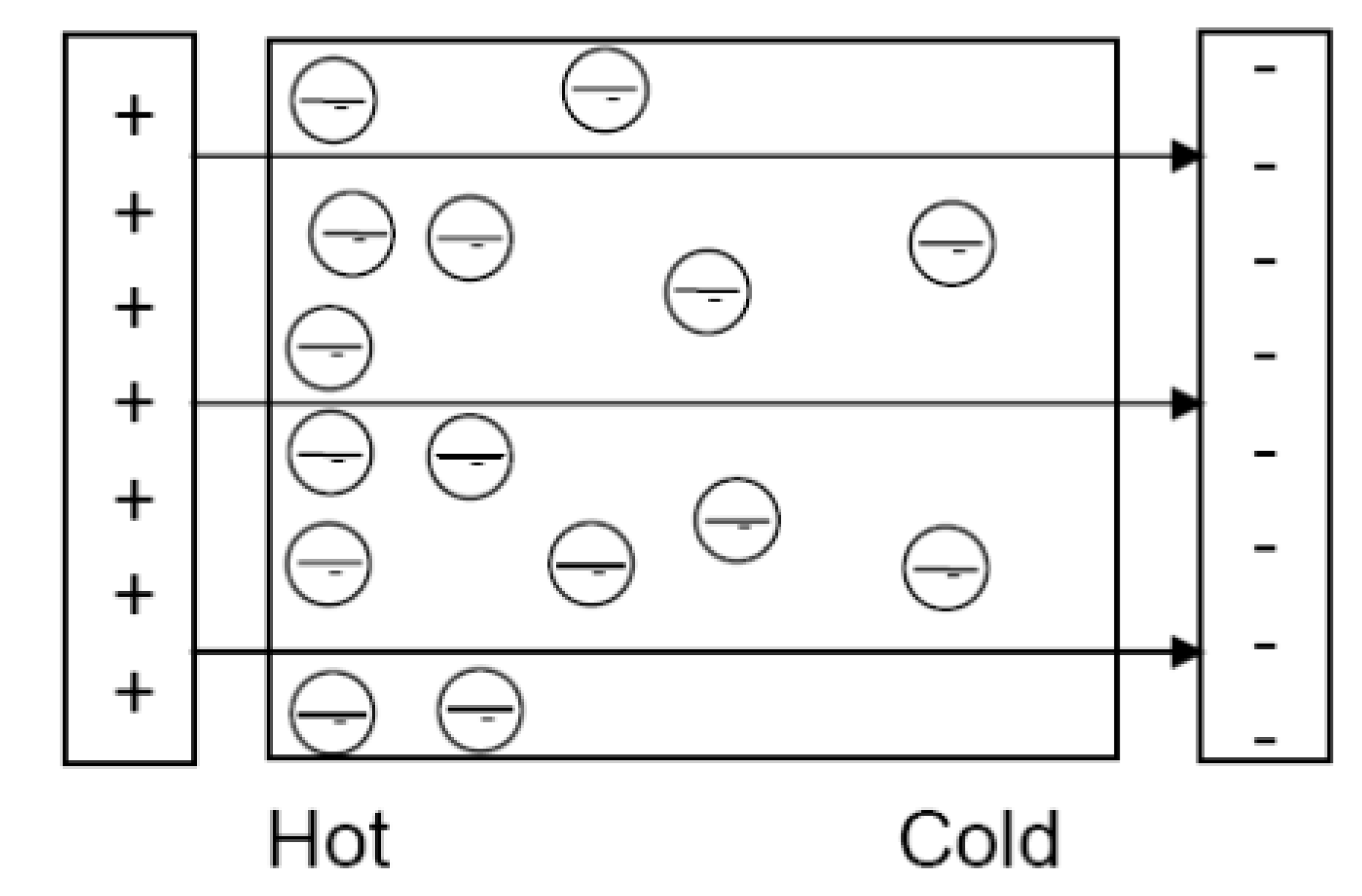
3.4. Diffusion-Diffusion Heat Flow Model
3.5. The Diffusion and Drift Model
3.6. Thermoelectric Materials
3.7. Hypothesized Thermoelectric Effects
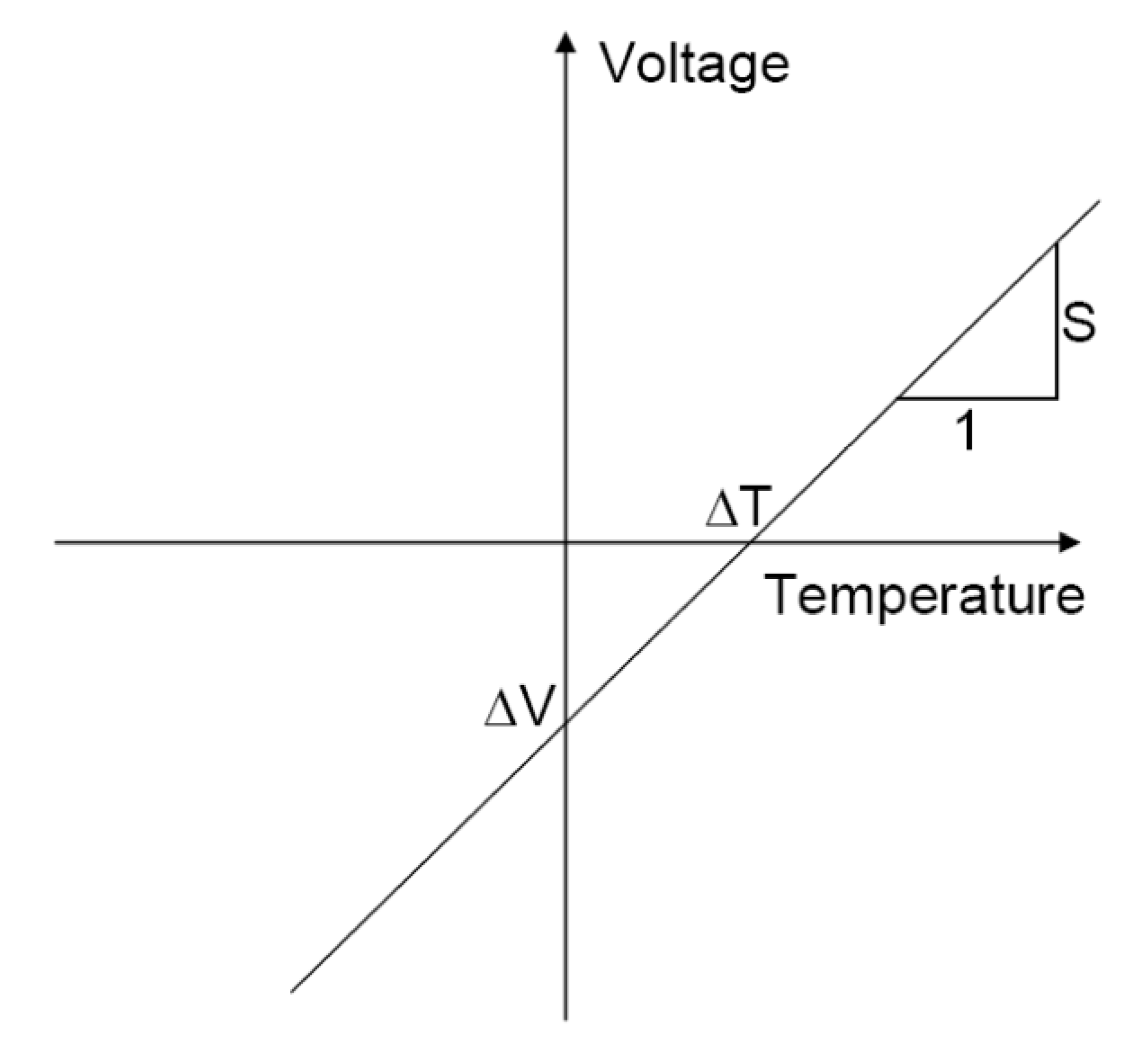
- An adiabatic temperature difference, ∆T, spontaneously develops across a potential barrier ∆E in a semiconductor in the absence of current or voltage across the barrier. This temperature difference is expected to be most pronounced in materials having a high ratio of thermal conductivity mediated by charge carriers to thermal conductivity mediated by heat phonons. A measure of this ratio is σ/K, the ratio of electrical conductivity to thermal conductivity.
- Conversely, a voltage ∆V is generated across the barrier if the temperature across the barrier is forcibly clamped to zero, thereby disturbing the temperature profile away from its equilibrium state. This voltage is proportional to the perturbation ∆Tp in the temperature away from its equilibrium state caused by the clamping, and its magnitude can be calculated using the Seebeck coefficient S. Thus:
- Conventional heat flow mediated by agents such as photons, phonons, or other particles not subjected to the force field. This heat flow occurs in the conventional direction, from hot to cold.
- Reverse heat flow by diffusion through the electrical carriers subjected to the force field. This heat flow must occur in the reverse direction to counterbalance the forward phonon heat flow and to satisfy the First Law. In high Z thermoelectric materials, this reverse heat flow gives rise to a temperature difference which is not fully cancelled by the forward heat flow because of the low coupling between the carriers and the phonons. This temperature difference becomes observable.
- Conventional heat flow due to an intentional thermal load such as a thermometer or a thermoelectric generator.
3.7. Heat Conductivity Equation
3.8. Seebeck Equation
4. Discussion of Experimental Results
4.1. Discussion of Experiment 1
4.2. Discussion of Experiment 2
4.3. Discussion of the Theory
4.4. Adiabatic Temperature Profile Simulation
4.5. Suggestion for Further Research
5. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Thomson, W. On the mechanical theory of thermo-electric currents. Trans. R. Soc. Edinburgh: Earth Sci. 1851, 3, 91–98. [Google Scholar]
- Rayleigh, L. On the thermodynamic efficiency of the thermopile. Philos. Mag. Ser. 5 1885, 20, 361–363. [Google Scholar] [CrossRef]
- Tolman, R.C.; Fine, P.C. On the irreversible production of entropy. Rev. Mod. Phys. 1948, 20, 51–57. [Google Scholar]
- Callen, H.B. The Application of onsager’s reciprocal relations to thermoelectric, thermomagnetic, and galvanomagnetic effects. Phys. Rev. 1948, 73, 1349–1358. [Google Scholar] [CrossRef]
- Callen, H.B. On the Theory of Irreversible Processes. Ph.D. Thesis, Massachusetts Institute of Technology—(M.I.T.), Cambridge, MA, USA, 1947. [Google Scholar]
- Callen, H.B.; Welton, T.A. Irreversibility and generalized noise. Phys. Rev. 1951, 83, 34–40. [Google Scholar] [CrossRef]
- Callen, H.B.; Greene, R.F. On a theorem of irreversible thermodynamics. Phys. Rev. 1952, 86, 702–710. [Google Scholar] [CrossRef]
- Callen, H.B. Thermodynamics; John Wiley & Sons, Inc.: New York, NY, USA, 1960. [Google Scholar]
- De Groot, S.R. Thermodynamics of Irreversible Processes; North-Holland Publishing Company: Amstedam, The Netherlands, 1963. [Google Scholar]
- Fieschi, R.; Groot, S.D.; Mazur, P.; Vlieger, J. Thermodynamical theory of galvanomagnetic and thermomagnetic phenomena II: Reciprocal relations for moving anisotropic mixtures. Physica 1954, 20, 245–258. [Google Scholar] [CrossRef]
- Fieschi, R.; Groot, S.D.; Mazur, P. Thermodynamical theory of galvanomagnetic and thermomagnetic phenomena. I: Reciprocal relations in anisotropic metals. Physica 1954, 20, 67–76. [Google Scholar] [CrossRef]
- Fieschi, R.; Groot, S.D.; Mazur, P. Thermodynamical theory of galvanomagnetic and thermomagnetic phenomena III: Explicit expressions for the measurable effects in isotropic metals. Physica 1954, 20, 259–273. [Google Scholar] [CrossRef]
- De Groot, S.; van Kampen, N. On the derivation of reciprocal relations between irreversible processes. Physica 1954, 21, 39–47. [Google Scholar] [CrossRef]
- Capeck, V.; Sheehan, D. Challenges to the Second Law of Thermodynamics; Springer: Norwell, MA, USA, 2005; ISBN 1-4020-3015-0(HB). pp. 202–203. [Google Scholar]
- Trupp, A. Energy, entropy: on the occasion of the 100th anniversary of Loschmidt’s death in 1895: Is Loschmidt’s greatest discovery waiting for its discovery? Phys. Essays 1999, 12, 614–628. [Google Scholar] [CrossRef]
- Martin, J.; Tritt, T.; Uher, C. High temperature seebeck coefficient metrology. J. Appl. Phys. 2010, 108, 121101. [Google Scholar] [CrossRef]
- Iwanaga, S.; Toberer, E.S.; LaLonde, A.; Snyder, G.J. A high temperature apparatus for measurement of the Seebeck coefficient. Rev. Sci. Instrum. 2011, 82, 063905-1–063905-6. [Google Scholar] [CrossRef] [PubMed]
- Snyder, Jeoffrey; Material Science, Caltech. Private communication on 20 January 2012.
- Snyder, Jeoffrey; Material Science, Caltech. Private communication on 9 April 2012.
- Von Zeghbroeck, B. Principles of Semiconductor Devices; Oxford University Press: New York, NY, USA, 2006; Available online: ecee.colorado.edu/~bart/book/book/ (accessed on 24 January 2013).
- Goupil, C.; Seifert, W.; Zabrocki, K.; Müller, E.; Snyder, J. Thermodynamics of thermoelectric phenomena and applications. Entropy 2011, 13, 1481–1517. [Google Scholar] [CrossRef]
- Peltier, J.C.A. Nouvelles expériences sur la caloricité des courants électriques. Ann. Chim. Phys. 1834, 56, 371–386. [Google Scholar]
- Seebeck, T.J. Ueber den Magnetismus der galvenischen Kette; Technical Report for the Royal Prussian Academy of Science: Berlin, Germany, 1821. [Google Scholar]
- Seebeck, T.J. Magnetische Polarisation der Metalle und Erze durch Temperatur-Differenz; Technical Report for the Royal Prussian Academy of Science: Berlin, Germany, 1823. [Google Scholar]
- Seebeck, T.J. Ueber die magnetische polarisation der metalle und erze durch temperatur-differenz. Annalen der Physik 1826, 82, 1–20. [Google Scholar] [CrossRef]
- In fact, Seebeck mistook the observed effect as a thermomagnetic coupling. The correct interpretation was later provided by Oersted.
- Jacobson, M.Z. Fundamentals of Atmospheric Modeling, 2nd ed.; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Donald Ahrens, C. Meteorology Today, 8th ed.; Brooks/Cole Publishing: Belmont, CA, USA, 2006. [Google Scholar]
- Glickman, T.S. Glossary of Meteorology, 2nd ed.; American Meteorological Society: Boston, MA, USA, 2000. [Google Scholar]
- Salomons, E.M. Computational Atmospheric Acoustics, 1st ed.; Springer: Secaucus, NJ, USA, 2001. [Google Scholar]
- Stull, R.B. An Introduction to Boundary Layer Meteorology, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Calvert, J.G. Glossary of Atmospheric Chemistry Terms Atmospheric Chemistry Division, National Center for Atmospheric Research, page 2171. In Pure&App/. Chem.; Vol. 62, No. 11; Boulder, CO, USA, 1990. [Google Scholar]
- Danielson, E.W.; Levin, J.; Abrams, E. Meteorology; McGraw Hill: Dubuque, Iowa, IA, USA, 2003. [Google Scholar]
- Fluid mechanics. In Course of Theoretical Physics, Volume 6; Landau, L.D.; Lifshitz, E.M. (Eds.) Pergamon Press: New York, NY, USA, 1982.
- Fronsdal, C. Heat and Gravitation. I. The Action Principle. 2011. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes. II. Phys. Rev. 1931, 38, 2265–2279. [Google Scholar] [CrossRef]
- Onsager, L. Theories and problems of liquid diffusion. Ann. N. Y. Acad. Sci. 1945, 46, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Callen, H.B. The application of Onsager’s reciprocal relations to thermoelectric, thermomagnetic, and galvanomagnetic effects. Phys. Rev. 1948, 73, 1349–1358. [Google Scholar] [CrossRef]
- Steinhart, J.S.; Hart, S.R. Calibration curves for thermistors. Deep Sea Res. Oceanogr. Abstr. 1968, 15, 497–503. [Google Scholar] [CrossRef]
- Entropic Power, Simulator. Available online: http://www.entropicpower.com/simulation (accessed on 24 October 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Levy, G. Thermoelectric Effects under Adiabatic Conditions. Entropy 2013, 15, 4700-4715. https://doi.org/10.3390/e15114700
Levy G. Thermoelectric Effects under Adiabatic Conditions. Entropy. 2013; 15(11):4700-4715. https://doi.org/10.3390/e15114700
Chicago/Turabian StyleLevy, George. 2013. "Thermoelectric Effects under Adiabatic Conditions" Entropy 15, no. 11: 4700-4715. https://doi.org/10.3390/e15114700
APA StyleLevy, G. (2013). Thermoelectric Effects under Adiabatic Conditions. Entropy, 15(11), 4700-4715. https://doi.org/10.3390/e15114700



