Reconsideration of Criteria and Modeling in Order to Optimize the Efficiency of Irreversible Thermomechanical Heat Engines
Abstract
:1. Introduction
2. General Model of the CARNOT Irreversible Engine
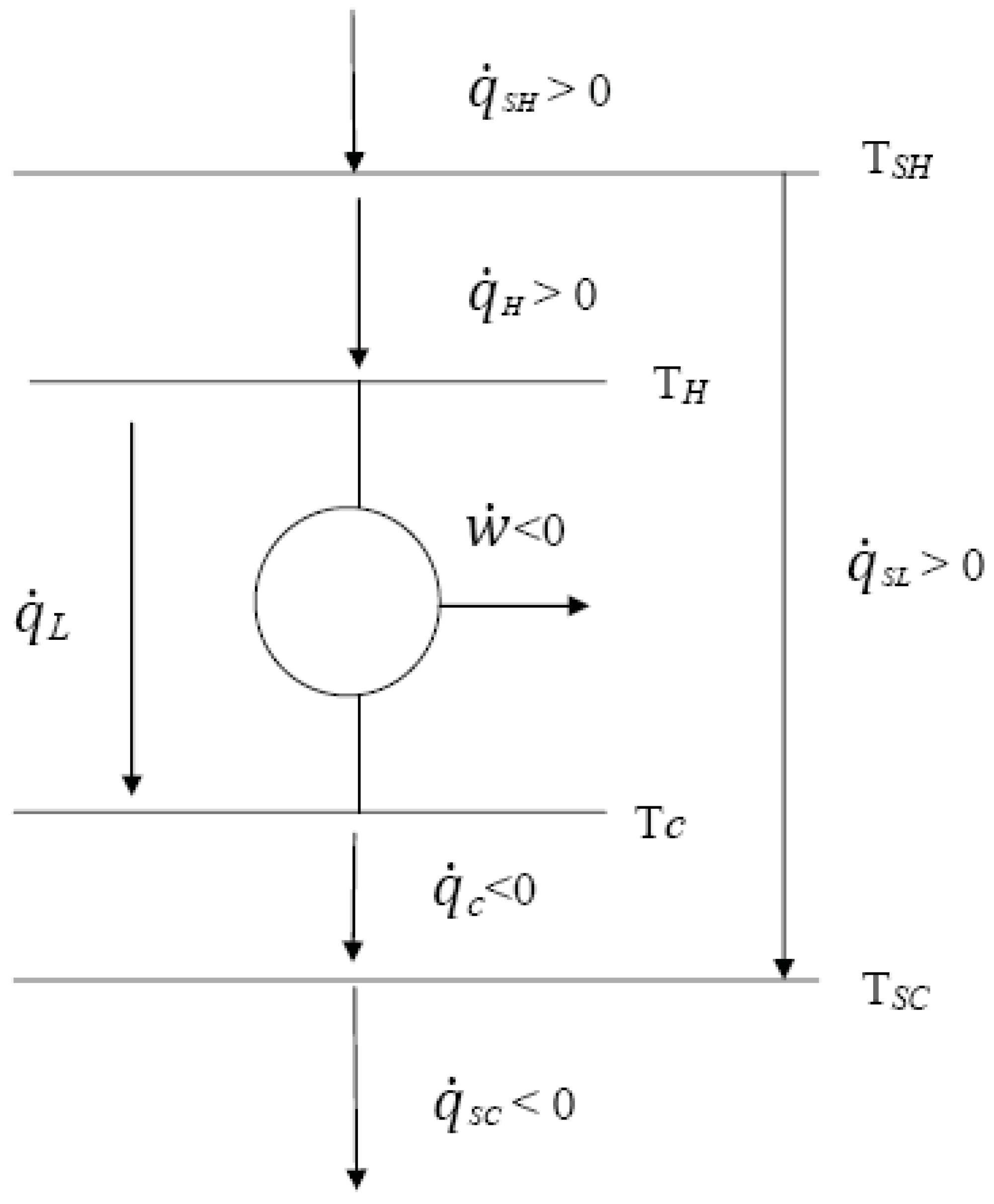
- KSL, generalized heat loss conductance,
- fSL(TSH, TSC), function characterizing the heat transfer.
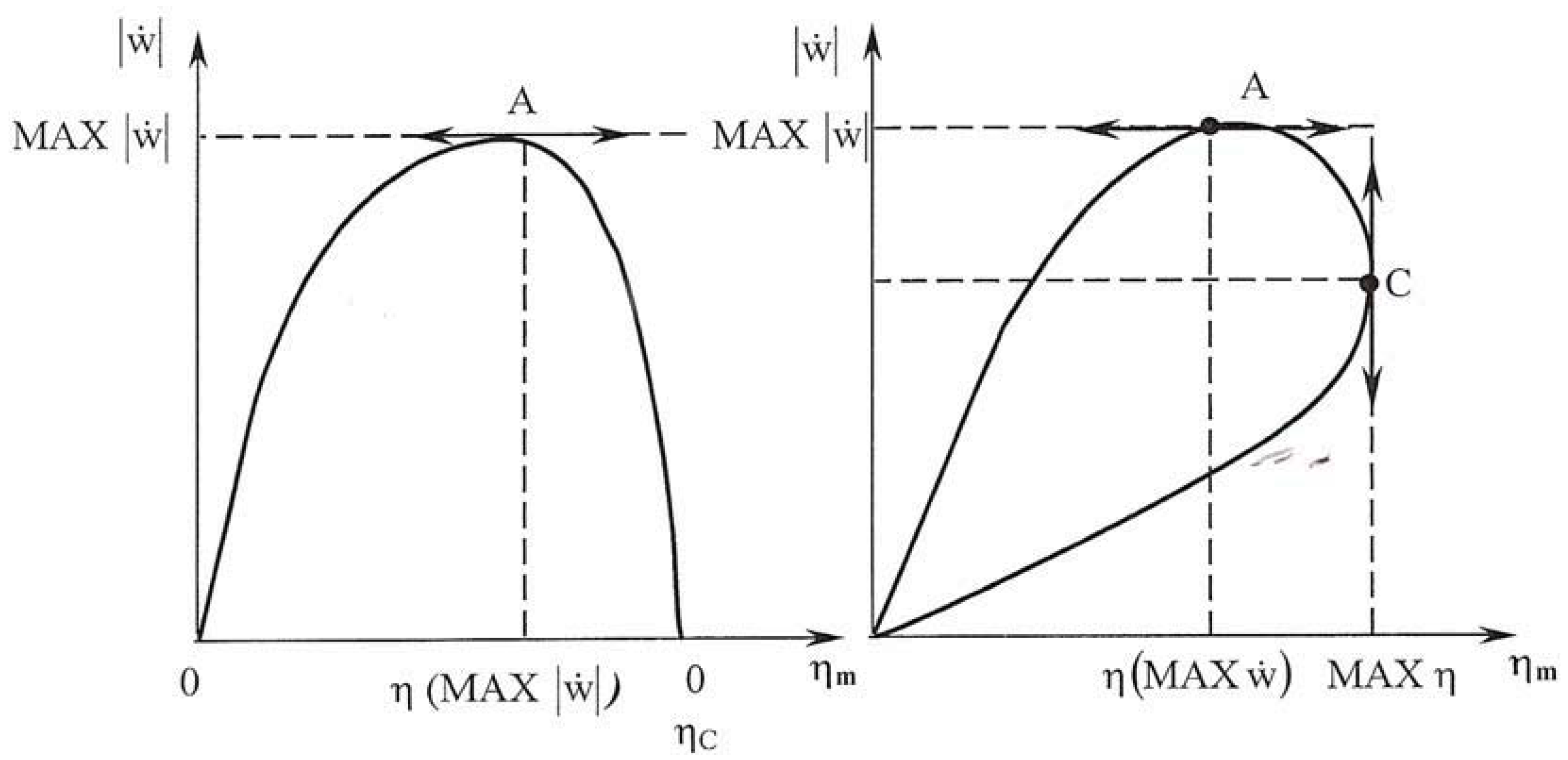
3. Maximum Efficiency Criterion
3.1. Developing the Criterion with the Degree of Freedom x
3.2. Developing the Criterion with Variationnal Calculus
4. Some Results and Discussion
4.1. Conditions Suggested in Paper [25]
4.1.1. Example 1: Linear Heat Transfer Laws
4.1.2. Example 2: Generalized Convective Laws
4.1.3. New Condition Deduced from the Criterion
4.2. Some Results Relative to the Criterion Revisited in Section 3.2
4.2.1. Case of a Carnot System with Linear Heat Transfer Laws
4.2.2. General Case of Total Entropy Rate
4.2.2.1. Endoreversible Engine, or = ct
4.2.2.2. Linear Approximation of or
- linear law:
- logarithmic law:
- phenomenological law:
4.3. Comparison of Extremum Condition of Efficiency with the one of Maximum Power, and Minimum Total Entropy Rate
4.3.1. Condition Relative to Maximization of Engine Power
4.3.2. Condition Relative to Minimization of the Total Entropy Rate
4.3.3. Comparison of the Obtained Three Necessary Conditions for Optimum Respectively of
5. Conclusions
- Reconsiders the conditions of various optimum proposed in the literature for an engine, and more precisely a thermomechanical one: the Carnot irreversible engine with heat losses in contact with two infinite reservoirs (source and sink), and steady state conditions. The engine connected to source and sink constitute the studied system. The obtained results differ significantly if we consider only the converter (engine). This precision appears essential.
- The three main objective functions explored are:
- -
- , maximum power
- -
- , maximum first law efficiency of the system
- -
- , minimum total generated entropy rate of the system. This new criterion could be an interesting one, regarding environmental and sustainable optimization.
- New expressions of the maximum first law efficiency criterion are given, for the engine (17), for the system (19), replacing the one given previously [10].
- Section 4 demonstrates that the results proposed by Aragon-Gonzales are particular (Section 4.1); if the entropy ratio method is used, new conditions for maximum first law system efficiency have been proposed.
- Section 4.2 particularizes the results to the entropy rate method proposed by the author, and summarized in a recent paper [26], using linear heat transfer laws at source and sink.
- Lastly, from a fundamental point of view, it has been shown in Section 4.3 that the extremum conditions or the three cited objectives occur at different analytical locations in term of variables (TH*, TC*); the corresponding value are obtained numerically, in general.
- The proposed method can be applied to other cycles, and more complete models; work is in progress in this direction.
References
- Maury, J.P. Carnot et la Machine à Vapeur; Presses Universitaires de France: Paris, France, 1987. [Google Scholar]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 1, 22–24. [Google Scholar] [CrossRef]
- Chambadal, P. Les Centrales Nucléaires; A. Colin: Paris, France, 1957. [Google Scholar]
- Novikov, I. The efficiency of atomic power stations (A review). Atom. Energ. 1957, 3, 3–11. [Google Scholar] [CrossRef]
- Wu, F.; Wu, C.; Guo, F.; Li, Q.; Chen, L. Optimization of a thermoacoustic engine with a complex heat transfer exponent. Entropy 2003, 5, 444–451. [Google Scholar] [CrossRef]
- Zheng, T.; Chen, L.; Sun, F.; Wu, C. Effect of heat leak and finite thermal capacity on the optimal configuration of a two-heat-reservoir heat engine for another linear heat transfer law. Entropy 2003, 5, 519–530. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, T.; Sun, F.; Wu, C. Optimal cooling load and COP relationship of a four-heat-reservoir endoreversible absorption refrigerator cycle. Entropy 2004, 6, 316–326. [Google Scholar] [CrossRef]
- Ladino-Luna, D. Van der Waals gas as working substance in a Curzon-Ahlborn-Novikov engine. Entropy 2005, 7, 108–121. [Google Scholar] [CrossRef]
- Ladino-Luna, D. On optimization of a non-endoreversible Curzon-Ahlborn cycle. Entropy 2007, 9, 186–197. [Google Scholar] [CrossRef]
- Feidt, M. Optimal thermodynamics—New upperbounds (A review). Entropy 2009, 11, 529–547. [Google Scholar] [CrossRef]
- Gordon, J.M. On optimizing maximum power heat engines. J. Appl. Phys. 1991, 69, 1–7. [Google Scholar] [CrossRef]
- Bejan, A. Theory of heat transfer irreversible power plant. Int. J. Heat Mass Transfer 1988, 31, 1211–1219. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Sun, F. The influence of internal heat leak on the power vs. efficiency characteristics of heat engines. Energ. Convers. Mgmt. 1997, 38, 1501–1507. [Google Scholar] [CrossRef]
- Moukalled, F.; Nuwayhid, R.Y.; Noueihed, N. The efficiency of endoreversible heat engines with heat leak. Int. J. Energ. Res. 1995, 19, 377–389. [Google Scholar] [CrossRef]
- Howe, J.P. The maximum power, hat demand and efficiency of a heat engine operating in steady state at less than Carnot efficiency. Energy 1982, 7, 401–402. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Klein, S.A.; Mitchell, J.W. Optimum heat power cycles for specified boundary conditions. J. Eng. Gas Turb. Power 1991, 113, 514–521. [Google Scholar] [CrossRef]
- Wu, C.; Kiang, R.L. Finite-time thermodynamic analysis of a Carnot engine with internal irreversibility. Energy 1992, 17, 1173–1178. [Google Scholar] [CrossRef]
- Chen, L.; Sun, F.; Wu, C. A generalized of real heat engines and its performance. J. Inst. Energ. 1996, 69, 214–222. [Google Scholar]
- Chen, L.; Sun, F.; Wu, C. Effect of heat transfer law on the performance of a generalized irreversible Carnot engine. J. Phys. D Appl. Phys. 1999, 32, 99–105. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, L.; Sun, F.; Wu, C. Optimal performance of a generalized irreversible Carnot engine. Appl. Energ. 2005, 81, 376–387. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Sun, F. Generalized irreversible heat-engine experiencing a complex heat-transfer law. Appl. Energ. 2008, 85, 52–60. [Google Scholar] [CrossRef]
- Chen, J. The maximum power output and maximum efficiency of an irreversible Carnot heat engine. J. Phys. D Appl. Phys. 1994, 27, 1144–1149. [Google Scholar] [CrossRef]
- Feidt, M.; Costea, M.; Petre, C.; Petrescu, S. Optimization of the direct Carnot cycle. Appl. Therm. Eng. 2007, 27, 829–839. [Google Scholar] [CrossRef]
- Bejan, A. Entropy Generation Trough Heat and Fluid Flow; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Aragon-Gonzales, G.; Canales-Palma, A.; León-Galicia, A.; Musharrafie-Martínez, M. A criterion to maximize the irreversible efficiency in heat engines. J. Phys. D Appl. Phys. 2003, 36, 280–287. [Google Scholar] [CrossRef]
- Feidt, M. Thermodynamics of energy systems and processes: A review and perspectives. In Proceedings of 2nd International Conference on Energy Conversion and Conservation, Hammamet, Tunisia, 22–25 April 2010.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feidt, M. Reconsideration of Criteria and Modeling in Order to Optimize the Efficiency of Irreversible Thermomechanical Heat Engines. Entropy 2010, 12, 2470-2484. https://doi.org/10.3390/e12122470
Feidt M. Reconsideration of Criteria and Modeling in Order to Optimize the Efficiency of Irreversible Thermomechanical Heat Engines. Entropy. 2010; 12(12):2470-2484. https://doi.org/10.3390/e12122470
Chicago/Turabian StyleFeidt, Michel. 2010. "Reconsideration of Criteria and Modeling in Order to Optimize the Efficiency of Irreversible Thermomechanical Heat Engines" Entropy 12, no. 12: 2470-2484. https://doi.org/10.3390/e12122470
APA StyleFeidt, M. (2010). Reconsideration of Criteria and Modeling in Order to Optimize the Efficiency of Irreversible Thermomechanical Heat Engines. Entropy, 12(12), 2470-2484. https://doi.org/10.3390/e12122470



