Cells 2021, 10(3), 565; https://doi.org/10.3390/cells10030565 - 5 Mar 2021
Cited by 64 | Viewed by 12654
Abstract
Myelin is the lipid-rich structure formed by oligodendrocytes (OLs) that wraps the axons in multilayered sheaths, assuring protection, efficient saltatory signal conduction and metabolic support to neurons. In the last few years, the impact of OL dysfunction and myelin damage has progressively received
[...] Read more.
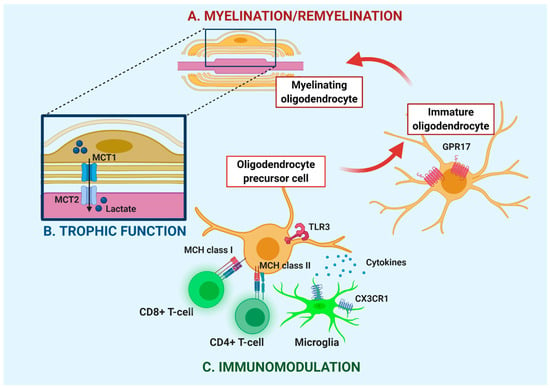
Myelin is the lipid-rich structure formed by oligodendrocytes (OLs) that wraps the axons in multilayered sheaths, assuring protection, efficient saltatory signal conduction and metabolic support to neurons. In the last few years, the impact of OL dysfunction and myelin damage has progressively received more attention and is now considered to be a major contributing factor to neurodegeneration in several neurological diseases, including amyotrophic lateral sclerosis (ALS). Upon OL injury, oligodendrocyte precursor cells (OPCs) of adult nervous tissue sustain the generation of new OLs for myelin reconstitution, but this spontaneous regeneration process fails to successfully counteract myelin damage. Of note, the functions of OPCs exceed the formation and repair of myelin, and also involve the trophic support to axons and the capability to exert an immunomodulatory role, which are particularly relevant in the context of neurodegeneration. In this review, we deeply analyze the impact of dysfunctional OLs in ALS pathogenesis. The possible mechanisms underlying OL degeneration, defective OPC maturation, and impairment in energy supply to motor neurons (MNs) have also been examined to provide insights on future therapeutic interventions. On this basis, we discuss the potential therapeutic utility in ALS of several molecules, based on their remyelinating potential or capability to enhance energy metabolism.
Full article
(This article belongs to the Special Issue The Contribution of Non-Neuronal Cells in Neurodegeneration: From Molecular Pathogenesis to Therapeutic Challenges)
►
Show Figures