Catalysts 2022, 12(1), 32; https://doi.org/10.3390/catal12010032 - 28 Dec 2021
Cited by 3 | Viewed by 2664
Abstract
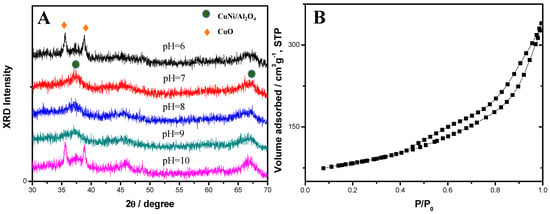
Mesoporous Cu-Ni/Al2O4 catalyst of high surface area (176 m2g−1) is synthesized through a simple hydrothermal reconstruction process by using low-cost activated alumina as the aluminate source without organic templates. The desired mesoporous structure of the catalyst
[...] Read more.
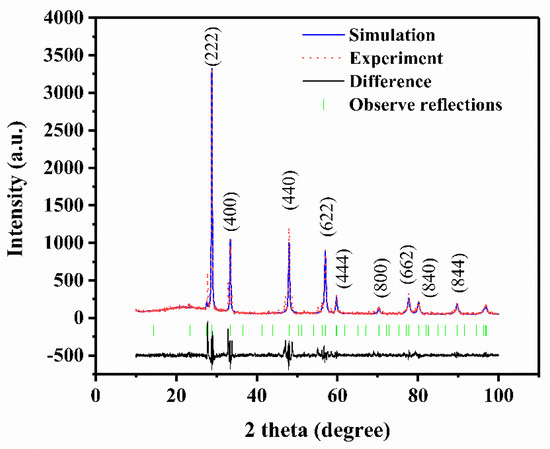
Mesoporous Cu-Ni/Al2O4 catalyst of high surface area (176 m2g−1) is synthesized through a simple hydrothermal reconstruction process by using low-cost activated alumina as the aluminate source without organic templates. The desired mesoporous structure of the catalyst is formed by the addition of Cu2+ and Ni2+ metal ions in the gel solution of the activated alumina followed by hydrothermal treatment at 70 °C and calcination at temperatures in the range of 600 to 800 °C. To consider the environmental concern, we found the concentration of the Cu2+ and Ni2+ ion in the residual filtrate is less than 0.1 ppm which satisfies the effluent standard in Taiwan (<1.0 ppm). The effects of the pH value, hydrothermal treatment time, and calcination temperature on the structure, morphology and surface area of the synthesized Cu-Ni/Al2O4 composites are investigated as well. In addition, the Cu-Ni/Al2O4 catalyst synthesized at pH 9.0 with a hydrothermal treatment time of 24 h and a calcination temperature of 600 °C is used for hydrogen production via the partial oxidation of methanol. The conversion efficiency is found to be >99% at a reaction temperature of around 315 °C, while the H2 yield is 1.99 mol H2/mol MeOH. The catalyst retains its original structure and surface area following the reaction process, and is thus inferred to have a good stability. Overall, the hydrothermal reconstruction route described herein is facile and easily extendable to the preparation of other mesoporous metal-alumina materials for catalyst applications.
Full article
(This article belongs to the Special Issue Catalytic Reforming for Syngas and H2 Productions)
►
Show Figures