Frank Reidy Research Center for Bioelectrics, 4211 Monarch Ways, Suite 300, Norfolk, VA 23508, USA
Cancers 2018, 10(4), 97; https://doi.org/10.3390/cancers10040097 - 30 Mar 2018
Cited by 28 | Viewed by 6977
Abstract
Nanopulse Stimulation (NPS) eliminates mouse and rat tumor types in several different animal models. NPS induces protective, vaccine-like effects after ablation of orthotopic rat N1-S1 hepatocellular carcinoma. Here we review some general concepts of NPS in the context of studies with mouse metastatic
[...] Read more.
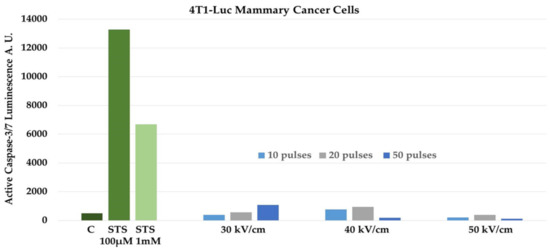
Nanopulse Stimulation (NPS) eliminates mouse and rat tumor types in several different animal models. NPS induces protective, vaccine-like effects after ablation of orthotopic rat N1-S1 hepatocellular carcinoma. Here we review some general concepts of NPS in the context of studies with mouse metastatic 4T1 mammary cancer showing that the postablation, vaccine-like effect is initiated by dynamic, multilayered immune mechanisms. NPS eliminates primary 4T1 tumors by inducing immunogenic, caspase-independent programmed cell death (PCD). With lower electric fields, like those peripheral to the primary treatment zone, NPS can activate dendritic cells (DCs). The activation of DCs by dead/dying cells leads to increases in memory effector and central memory T-lymphocytes in the blood and spleen. NPS also eliminates immunosuppressive cells in the tumor microenvironment and blood. Finally, NPS treatment of 4T1 breast cancer exhibits an abscopal effect and largely prevents spontaneous metastases to distant organs. NPS with fast rise–fall times and pulse durations near the plasma membrane charging time constant, which exhibits transient, high-frequency components (1/time = Hz), induce responses from mitochondria, endoplasmic reticulum, and nucleus. Such effects may be responsible for release of danger-associated molecular patterns, including ATP, calreticulin, and high mobility group box 1 (HMBG1) from 4T1-Luc cells to induce immunogenic cell death (ICD). This likely leads to immunity and the vaccine-like response. In this way, NPS acts as a unique onco-immunotherapy providing distinct therapeutic advantages showing possible clinical utility for breast cancers as well as for other malignancies.
Full article
(This article belongs to the Special Issue Electric Field Based Therapies for Cancer: A Selection of Papers from the 2nd World Congress on Electroporation)
▼
Show Figures