Pharmaceutics 2023, 15(3), 757; https://doi.org/10.3390/pharmaceutics15030757 - 24 Feb 2023
Cited by 18 | Viewed by 2488
Abstract
This paper focuses on recent advancements in the development of 4D printed drug delivery systems (DDSs) for the intravesical administration of drugs. By coupling the effectiveness of local treatments with major compliance and long-lasting performance, they would represent a promising innovation for the
[...] Read more.
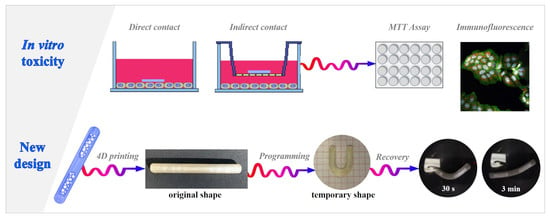
This paper focuses on recent advancements in the development of 4D printed drug delivery systems (DDSs) for the intravesical administration of drugs. By coupling the effectiveness of local treatments with major compliance and long-lasting performance, they would represent a promising innovation for the current treatment of bladder pathologies. Being based on a shape-memory pharmaceutical-grade polyvinyl alcohol (PVA), these DDSs are manufactured in a bulky shape, can be programmed to take on a collapsed one suitable for insertion into a catheter and re-expand inside the target organ, following exposure to biological fluids at body temperature, while releasing their content. The biocompatibility of prototypes made of PVAs of different molecular weight, either uncoated or coated with Eudragit®-based formulations, was assessed by excluding relevant in vitro toxicity and inflammatory response using bladder cancer and human monocytic cell lines. Moreover, the feasibility of a novel configuration was preliminarily investigated, targeting the development of prototypes provided with inner reservoirs to be filled with different drug-containing formulations. Samples entailing two cavities, filled during the printing process, were successfully fabricated and showed, in simulated urine at body temperature, potential for controlled release, while maintaining the ability to recover about 70% of their original shape within 3 min.
Full article
(This article belongs to the Special Issue Local Drug Delivery System)
►
Show Figures