Department of Medical, Surgical and Neurological Sciences, University of Siena, 53100 Siena, Italy
Int. J. Mol. Sci. 2017, 18(4), 775; https://doi.org/10.3390/ijms18040775 - 6 Apr 2017
Cited by 20 | Viewed by 8080
Abstract
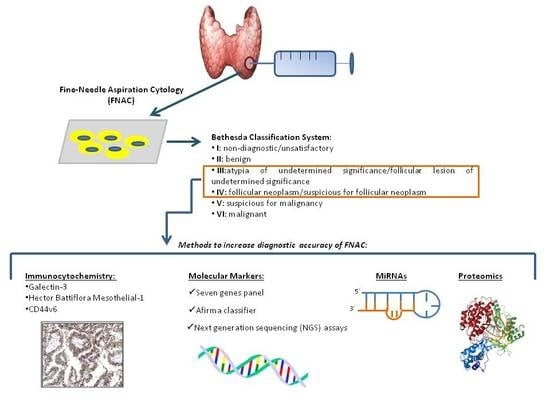
Fine needle aspiration cytology (FNAC) represents the gold standard for determining the nature of thyroid nodules. It is a reliable method with good sensitivity and specificity. However, indeterminate lesions remain a diagnostic challenge and researchers have contributed molecular markers to search for in
[...] Read more.
Fine needle aspiration cytology (FNAC) represents the gold standard for determining the nature of thyroid nodules. It is a reliable method with good sensitivity and specificity. However, indeterminate lesions remain a diagnostic challenge and researchers have contributed molecular markers to search for in cytological material to refine FNAC diagnosis and avoid unnecessary surgeries. Nowadays, several “home-made” methods as well as commercial tests are available to investigate the molecular signature of an aspirate. Moreover, other markers (i.e., microRNA, and circulating tumor cells) have been proposed to discriminate benign from malignant thyroid lesions. Here, we review the literature and provide data from our laboratory on mutational analysis of FNAC material and circulating microRNA expression obtained in the last 6 years.
Full article
(This article belongs to the Special Issue Current Knowledge in Thyroid Cancer—From Bench to Bedside)
▼
Show Figures