In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites
Abstract
:1. Introduction
2. The Chemistry of In Situ Reactions
2.1. Sol–Gel Process and the Formation of the Inorganic Network
2.2. Formation of the Organic Network and Crosslinks between the Organic and Inorganic Components in Hybrids
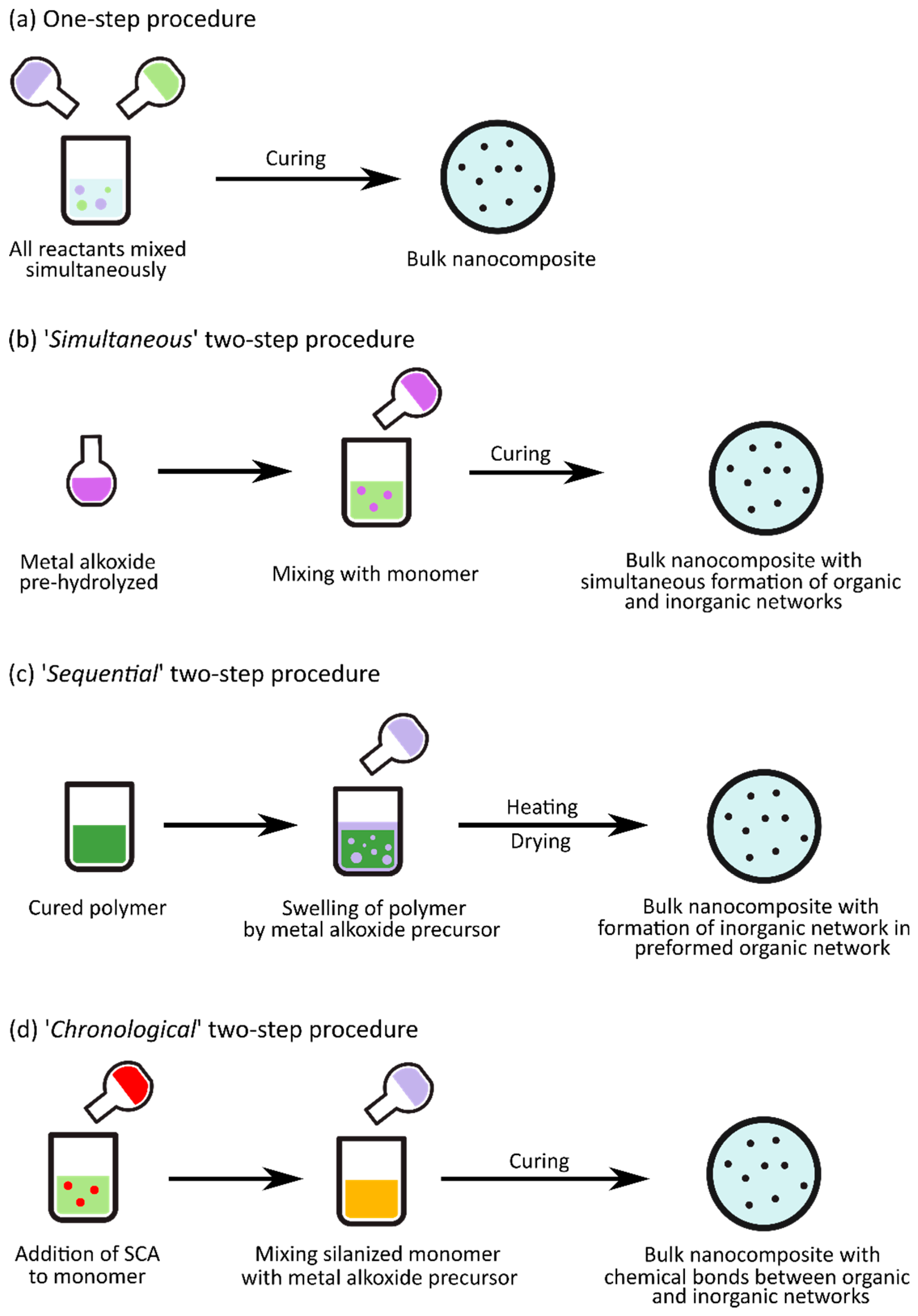
3. Nanocomposite Fabrication via Sol–Gel Processes
3.1. Epoxy Nanocomposites
3.1.1. Effect of Synthesis Procedure and pH on the Structure and Morphology of Nanocomposites
3.1.2. The Effect of Silane Coupling Agents
3.1.3. Application of Ionic Liquids in the Synthesis Procedure
3.2. Polysiloxane Nanocomposites
3.2.1. In Situ Synthesis Procedures Using a Chelating Agent for the Transition Metal Alkoxides
3.2.2. In Situ Synthesis Procedures without Chelating Agents
3.2.3. PDMS–SiO2 Nanocomposites by Swelling Techniques
4. Alternative Methods for the In Situ Preparation of Nanocomposites
5. Prospects of In Situ Hybrid Materials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F. Design of Hybrid Organic-Inorganic Materials Synthesized Via Sol-Gel Chemistry. New J. Chem. 1994, 18, 1007–1047. [Google Scholar] [CrossRef]
- Mark, J.E.; Pan, S.-J. Reinforcement of polydimethylsiloxane networks by in-situ precipitation of silica: A new method for preparation of filled elastomers. Die Makromol. Chem. Rapid Commun. 1982, 3, 681–685. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Jones, C.K. Novel poly(n-butyl methacrylate)/titanium oxide alloys produced by te sol–gel process for titanium alkoxides. J. Appl. Polym. Sci. 1990, 40, 1401–1420. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid Sol-Gel-Derived Polymers: Applications of Multifunctional Materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Gallon, B.J.; Kojima, R.W.; Kaner, R.B.; Diaconescu, P.L. Palladium nanoparticles supported on polyaniline nanofibers as a semi-heterogeneous catalyst in water. Angew. Chem. Int. Ed. 2007, 46, 7251–7254. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Q.; Xia, H. Template synthesis of polyaniline/TiO2 bilayer microtubes. Synth. Met. 2004, 146, 37–42. [Google Scholar] [CrossRef]
- Leong, W.L.; Lee, P.S.; Lohani, A.; Lam, Y.M.; Chen, T.; Zhang, S.; Dodabalapur, A.; Mhaisalkar, S.G. Non-volatile organic memory applications enabled by in situ synthesis of gold nanoparticles in a self-assembled block copolymer. Adv. Mater. 2008, 20, 2325–2331. [Google Scholar] [CrossRef]
- Pothukuchi, S.; Li, Y.; Wong, C.P. Development of a novel polymer-metal nanocomposite obtained through the route of in situ reduction for integral capacitor application. J. Appl. Polym. Sci. 2004, 93, 1531–1538. [Google Scholar] [CrossRef]
- Dalod, A.; Grendal, O.; Blichfeld, A.; Furtula, V.; Pérez, J.; Henriksen, L.; Grande, T.; Einarsrud, M.-A. Structure and Optical Properties of Titania-PDMS Hybrid Nanocomposites Prepared by In Situ Non-Aqueous Synthesis. Nanomaterials 2017, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Lü, C.; Yang, B. High refractive index organic–inorganic nanocomposites: Design, synthesis and application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Nelson, J.K.; Fothergill, J.C. Internal charge behaviour of nanocomposites. Nanotechnology 2004, 15, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Kochetov, R.; Andritsch, T.; Morshuis, P.H.F.; Smit, J.J. Anomalous Behaviour of the Dielectric Spectroscopy Response of Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 107–117. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, M.J. Dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 12–23. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.-C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Nelson, J.K.; Nelson, J.K. Dielectric Polymer Nanocomposites; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-1590-0. [Google Scholar]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Caseri, W.R. In situ synthesis of polymer-embedded nanostructures. In Nanocomposites: In Situ Synthesis of Polymer-Embedded Nanostructures; Nicolais, L., Catotenuto, G., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 45–72. [Google Scholar]
- Caseri, W.R. Nanocomposites of polymers and inorganic particles: Preparation, structure and properties. Mater. Sci. Technol. 2006, 22, 807–817. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Baek, J.B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef] [Green Version]
- Althues, H.; Henle, J.; Kaskel, S. Functional inorganic nanofillers for transparent polymers. Chem. Soc. Rev. 2007, 36, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol-Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njugana, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Bounor-Legaré, V.; Cassagnau, P. In situ synthesis of organic-inorganic hybrids or nanocomposites from sol-gel chemistry in molten polymers. Prog. Polym. Sci. 2014, 39, 5050–5056. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, R.; Tong, M.; Su, Y.; Huang, M. In Situ Solvothermal Synthesis and Characterization of Transparent Epoxy/TiO2 Nanocomposites. J. Appl. Polym. Sci. 2012, 125, 1152–1160. [Google Scholar] [CrossRef]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Chen, Q.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and mechanical properties of PDMS-modified CaO-SiO2-TiO2 hybrids prepared by sol-gel process. J. Biomed. Mater. Res. 2000, 51, 605–611. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Polymer sol-gel synthesis of hybrid nanocomposites. Colloid J. 2005, 67, 658–677. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C. Sol-gel chemistry. J. Non-Cryst. Solids 1992, 145, 11–19. [Google Scholar] [CrossRef]
- Yu, W.; Fu, J.; Dong, X.; Chen, L.; Jia, H.; Shi, L. Highly populated and nearly monodispersed nanosilica particles in an organic medium and their epoxy nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 8897–8906. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.N.; Raval, H.M. Synthesis of organic-inorganic hybrids via the non-hydrolytic sol-gel process. Chem. Mater. 2001, 13, 3396–3403. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Barnes, C.; Pinkas, J. The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review. Catalysts 2017, 7, 168. [Google Scholar] [CrossRef]
- Ogoshi, T.; Chujo, Y. Organic-inorganic polymer hybrids prepared by the sol-gel method. Compos. Interfaces 2005, 11, 539–566. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol–Gel Processing; Academic Press, Inc.: Cambridge, MA, USA, 1990; ISBN 9780080571034. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G.; Balsubramaniam, R. Callister’s Materials Science and Engineering, 8th ed.; Wiley India: New Delhi, India, 2010. [Google Scholar]
- Solomons, G.; Fryhle, C. Organic Chemistry, 10th ed.; Wiley: New Delhi, India, 2009; ISBN 0470556595. [Google Scholar]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Springer-Verlag US: Boston, MA, USA, 1991; ISBN 978-1-4899-2070-6. [Google Scholar]
- Plueddemann, E.P. Adhesion Through Silane Coupling Agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M. Dispersion of Surface Modified Nanostructure Zinc Oxide in Optically Active Poly(Amide-Imide) Containing Pyromellitoyl-bis-l-isoleucine Segments: Nanocomposite Preparation and Morphological Investigation. Polym. Plast. Technol. Eng. 2012, 51, 1106–1112. [Google Scholar] [CrossRef]
- Dalod, A.R.M.; Grendal, O.G.; Skjærvø, S.L.; Inzani, K.; Selbach, S.M.; Henriksen, L.; van Beek, W.; Grande, T.; Einarsrud, M.-A. Controlling Oriented Attachment and in Situ Functionalization of TiO2 Nanoparticles During Hydrothermal Synthesis with APTES. J. Phys. Chem. C 2017, 121, 11897–11906. [Google Scholar] [CrossRef]
- Dalod, A.R.M.; Henriksen, L.; Grande, T.; Einarsrud, M.-A. Functionalized TiO2 nanoparticles by single-step hydrothermal synthesis: The role of the silane coupling agents. Beilstein J. Nanotechnol. 2017, 8, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-T.; Li, Z.-J.; Liang, W. Preparation and characterization of epoxy/SiO2 nanocomposites by cationic photopolymerization and sol–gel process. Polym. Adv. Technol. 2013, 25, 173–178. [Google Scholar] [CrossRef]
- Yang, C.-F.; Wang, L.-F.; Wu, S.-M.; Su, C.-C. Characterization and Curing Kinetics of Epoxy/Silica Nano-Hybrids. Materials 2015, 8, 7032–7040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, J.; Liu, P.; Cai, Y. One-step synthesis of improved silica/epoxy nanocomposites with inorganic-organic hybrid network. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Afzal, A.; Siddiqi, H.M. A comprehensive study of the bicontinuous epoxy-silica hybrid polymers: I. Synthesis, characterization and glass transition. Polymer 2011, 52, 1345–1355. [Google Scholar] [CrossRef]
- Nazir, T.; Afzal, A.; Siddiqi, H.M.; Ahmad, Z.; Dumon, M. Thermally and mechanically superior hybrid epoxy-silica polymer films via sol-gel method. Prog. Org. Coat. 2010, 69, 100–106. [Google Scholar] [CrossRef]
- Siddabattuni, S.; Schuman, T.P.; Dogan, F. Dielectric Properties of Polymer−Particle Nanocomposites Influenced by Electronic Nature of Filler Surfaces. ACS Appl. Mater. Interfaces 2013, 5, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.J.; Liu, D.W.; Jackson, C.L.; Barnes, J.D. Epoxy/SiO2 interpenetrating polymer networks. Polym. Adv. Technol. 1996, 7, 333–339. [Google Scholar] [CrossRef]
- Matějka, L.; Pleštil, J.; Dušek, K. Structure evolution in epoxy–silica hybrids: Sol–gel process. J. Non-Cryst. Solids 1998, 226, 114–121. [Google Scholar] [CrossRef]
- Matějka, L.; Dušek, K.; Pleštil, J.; Kříž, J.; Lednický, F. Formation and structure of the epoxy-silica hybrids. Polymer 1999, 40, 171–181. [Google Scholar] [CrossRef]
- Matejka, L.; Dukh, O.; Kolařík, J. Reinforcement of crosslinked rubbery epoxies by in-situ formed silica. Polymer 2000, 41, 1449–1459. [Google Scholar] [CrossRef]
- Chiang, C.L.; Ma, C.C.M. Synthesis, characterization and thermal properties of novel epoxy containing silicon and phosphorus nanocomposites by sol-gel method. Eur. Polym. J. 2002, 38, 2219–2224. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, S.L.C. Preparation of epoxy/silica and epoxy/titania hybrid resists via a sol-gel process for nanoimprint lithography. J. Phys. Chem. C 2010, 114, 2179–2183. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Amerio, E.; Bongiovanni, R.; Priola, A. Preparation and Characterization of Nanostructured TiO2/Epoxy Polymeric Films. Macromol. Mater. Eng. 2006, 291, 517–523. [Google Scholar] [CrossRef]
- Guan, C.; Lü, C.L.; Liu, Y.F.; Yang, B. Preparation and characterization of high refractive index thin films of TiO2/epoxy resin nanocomposites. J. Appl. Polym. Sci. 2006, 102, 1631–1636. [Google Scholar] [CrossRef]
- Donato, R.K.; Perchacz, M.; Ponyrko, S.; Donato, K.Z.; Schrekker, H.S.; Beneš, H.; Matějka, L. Epoxy–silica nanocomposite interphase control using task-specific ionic liquids via hydrolytic and non-hydrolytic sol–gel processes. RSC Adv. 2015, 5, 91330–91339. [Google Scholar] [CrossRef]
- Donato, R.K.; Matějka, L.; Schrekker, H.S.; Pletil, J.; Jigounov, A.; Brus, J.; Slouf, M. The multifunctional role of ionic liquids in the formation of epoxy-silica nanocomposites. J. Mater. Chem. 2011, 21, 13801–13810. [Google Scholar] [CrossRef]
- Donato, R.K.; Donato, K.Z.; Schrekker, H.S.; Matějka, L. Tunable reinforcement of epoxy-silica nanocomposites with ionic liquids. J. Mater. Chem. 2012, 22, 9939–9948. [Google Scholar] [CrossRef]
- Glaser, R.H.; Wilkes, G.L. Structure property behavior of polydimethylsiloxane and poly(tetramethylene oxide) modified TEOS based sol-gel materials. V. Effect of titaniumisopropoxide incorporation. Polym. Bull. 1988, 19, 51–57. [Google Scholar] [CrossRef]
- Shindou, T.; Katayama, S.; Yamada, N.; Kamiya, K. Effect of composition on surface properties of polydimethylsiloxane-based inorganic/organic hybrid films. J. Sol-Gel Sci. Technol. 2004, 30, 229–237. [Google Scholar] [CrossRef]
- Lu, Q.; Mullins, M.E. In situ Synthesis of High Refractive Index PDMS/Metal Oxide Nanocomposites. MRS Proc. 2012, 1400. [Google Scholar] [CrossRef]
- Wu, L.Y.L.; Tan, G.H.; Zeng, X.T.; Li, T.H.; Chen, Z. Synthesis and characterization of transparent hydrophobic sol-gel hard coatings. J. Sol-Gel Sci. Technol. 2006, 38, 85–89. [Google Scholar] [CrossRef]
- Yamada, N.; Yoshinaga, I.; Katayama, S. Formation behavior and optical properties of transparent inorganic-organic hybrids prepared from metal alkoxides and polydimethylsiloxane. J. Sol-Gel Sci. Technol. 2000, 17, 123–130. [Google Scholar] [CrossRef]
- Yamada, N.; Yoshinaga, I.; Katayama, S. Synthesis and dynamic mechanical behaviour of inorganic–organic hybrids containing various inorganic components. J. Mater. Chem. 1997, 7, 1491–1495. [Google Scholar] [CrossRef]
- Yamada, N.; Yoshinaga, I.; Katayama, S. Processing and properties of inorganic-organic hybrids containing various inorganic components. J. Sol-Gel Sci. Technol. 1999, 13, 445–449. [Google Scholar] [CrossRef]
- Katayama, S.; Kubo, Y.; Yamada, N. Characterization and mechanical properties of flexible dimethylsiloxane-based inorganic/organic hybrid sheets. J. Am. Ceram. Soc. 2002, 85, 1157–1163. [Google Scholar] [CrossRef]
- Carlos Almeida, J.; Castro, A.G.B.; Miranda Salvado, I.M.; Margaça, F.M.A.; Vaz Fernandes, M.H. A new approach to the preparation of PDMS–SiO2 based hybrids—A structural study. Mater. Lett. 2014, 128, 105–109. [Google Scholar] [CrossRef]
- Julián, B.; Gervais, C.; Cordoncillo, E.; Escribano, P.; Babonneau, F.; Sanchez, C. Synthesis and characterization of transparent PDMS-metal-oxo based organic-inorganic nanocomposites. Chem. Mater. 2003, 15, 3026–3034. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P.; Bokobza, L. Glass transition and molecular dynamics in poly(dimethylsiloxane)/silica nanocomposites. Polymer 2005, 46, 6001–6008. [Google Scholar] [CrossRef]
- Dewimille, L.; Bresson, B.; Bokobza, L. Synthesis, structure and morphology of poly(dimethylsiloxane) networks filled with in situ generated silica particles. Polymer 2005, 46, 4135–4143. [Google Scholar] [CrossRef]
- Yuan, Q.W.; Mark, J.E. Reinforcement of poly(dimethylsiloxane) networks by blended andin-situgenerated silica fillers having various sizes, size distributions, and modified surfaces. Macromol. Chem. Phys. 1999, 200, 206–220. [Google Scholar] [CrossRef]
- Breiner, J.M.; Mark, J.E.; Beaucage, G. Dependence of silica particle sizes on network chain lengths, silica contents, and catalyst concentrations in in situ-reinforced polysiloxane elastomers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1421–1427. [Google Scholar] [CrossRef]
- Rajan, G.S.; Sur, G.S.; Mark, J.E.; Schaefer, D.W.; Beaucage, G. Preparation and characterization of some unusually transparent poly(dimethylsiloxane) nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1897–1901. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, H.; Xiao, B.; Yan, L.; Lv, H.; Jiang, B. Sol-Gel Preparation of PDMS/Silica Hybrid Antireflective Coatings with Controlled Thickness and Durable Antireflective Performance. J. Phys. Chem. C 2010, 114, 19979–19983. [Google Scholar] [CrossRef]
- Donato, R.K.; Lavorgna, M.; Musto, P.; Donato, K.Z.; Jager, A.; Štěpánek, P.; Schrekker, H.S.; Matějka, L. The role of ether-functionalized ionic liquids in the sol-gel process: Effects on the initial alkoxide hydrolysis steps. J. Colloid Interface Sci. 2015, 447, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Seyferth, D. Dimethyldichlorosilane and the Direct Synthesis of Methylchlorosilanes. The Key to the Silicones Industry. Organometallics 2001, 20, 4978–4992. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A.A. Modification of polysiloxane polymers for biomedical applications: A review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Kumar, B.V.M.; Kim, Y.W. Processing of polysiloxane-derived porous ceramics: A review. Sci. Technol. Adv. Mater. 2010, 11. [Google Scholar] [CrossRef]
- Kataoka, T.; Ueda, S. Viscosity–molecular weight relationship for polydimethylsiloxane. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 317–322. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P.; Bokobza, L. Modified chain dynamics in poly(dimethylsiloxane)/silica nanocomposites. J. Non-Cryst. Solids 2006, 352, 4969–4972. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P. Glass transition and segmental dynamics in poly(dimethylsiloxane)/silica nanocomposites studied by various techniques. J. Non-Cryst. Solids 2007, 353, 4344–4352. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.Y.; Hong, L. In situ preparation of poly(ethylene oxide)-SiO2 composite polymer electrolytes. J. Power Sources 2004, 129, 303–311. [Google Scholar] [CrossRef]
- Bahloul, W.; Bounor-Legaré, V.; David, L.; Cassagnau, P. Morphology and viscoelasticity of PP/TiO2 nanocomposites prepared by in situ sol-gel method. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1213–1222. [Google Scholar] [CrossRef]
- Bahloul, W.; Oddes, O.; Bounor-Legaré, V.; Mélis, F.; Cassagnau, P.; Vergnes, B. Reactive extrusion processing of polypropylene/TiO2 nanocomposites by in situ synthesis of the nanofillers: Experiments and modeling. AIChE J. 2011, 57, 2174–2184. [Google Scholar] [CrossRef]
- Jain, S.; Goossens, H.; Picchioni, F.; Magusin, P.; Mezari, B.; Van Duin, M. Synthetic aspects and characterization of polypropylene-silica nanocomposites prepared via solid-state modification and sol-gel reactions. Polymer 2005, 46, 6666–6681. [Google Scholar] [CrossRef]
- Chen, Y.; Iroh, J.O. Synthesis and Characterization of Polymide/Silica Hybrid Composites. Chem. Mater. 1999, 11, 1218–1222. [Google Scholar] [CrossRef]
- González, M.; Soares, B.G.; Magioli, M.; Marins, J.A.; Rieumont, J. Facile method for synthesis of polyaniline/silica hybrid composites by simultaneous sol-gel process and “in situ” polymerization of aniline. J. Sol-Gel Sci. Technol. 2012, 63, 373–381. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, B.; Wang, F.; Ding, Y.; Zhang, S.; Yang, M. In situ synthesis of ZnO nanocrystal/PET hybrid nanofibers via electrospinning. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1360–1368. [Google Scholar] [CrossRef]
- Balci, M.H.; Aas, L.M.S.; Kildemo, M.; Sæterli, R.; Holmestad, R.; Lindgren, M.; Grande, T.; Einarsrud, M.A. White light emitting silicon nano-crystals-polymeric hybrid films prepared by single batch solution based method. Thin Solid Films 2016, 603, 126–133. [Google Scholar] [CrossRef]
- Fearon, F.W.G.; Young, J.C. Reaction of triphenylsilyl halides with sodium naphthalenide. J. Chem. Soc. B Phys. Org. 1971, 272–276. [Google Scholar] [CrossRef]
- Balci, M.H.; Maria, J.; Vullum-Bruer, F.; Lindgren, M.; Grande, T.; Einarsrud, M.A. Synthesis of Monodisperse Silicon Quantum Dots Through a K-Naphthalide Reduction Route. J. Clust. Sci. 2012, 23, 421–435. [Google Scholar] [CrossRef]
- Sletnes, M.; Maria, J.; Grande, T.; Lindgren, M.; Einarsrud, M.A. Octoxy capped Si nanoparticles synthesized by homogeneous reduction of SiCl4 with crown ether alkalide. Dalt. Trans. 2014, 43, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Stille, J.K. Diels-alder polymerization. In Fortschritte Der Hochpolymeren-Forschung; Springer: Berlin/Heidelberg, Germany, 1961; Volume 3, pp. 48–58. [Google Scholar] [CrossRef]
- Sohn, H.; Huddleston, R.R.; Powell, D.R.; West, R.; Oka, K.; Yonghua, X. An electroluminescent polysilole and some dichlorooligosiloles. J. Am. Chem. Soc. 1999, 121, 2935–2936. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P. Preparation and characterizations of a new PS/TiO2 hybrid membranes by sol–gel process. Polymer 2006, 47, 2683–2688. [Google Scholar] [CrossRef]
- Bounor-Legaré, V.; Angelloz, C.; Blanc, P.; Cassagnau, P.; Michel, A. A new route for organic-inorganic hybrid material synthesis through reactive processing without solvent. Polymer 2004, 45, 1485–1493. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. In situ polymerization of silicic acid in polyethylene-octene elastomer: Properties and characterization of the hybrid nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 351–359. [Google Scholar] [CrossRef]
- He, J.-P.; Li, H.-M.; Wang, X.-Y.; Gao, Y. In situ preparation of poly(ethylene terephthalate)–SiO2 nanocomposites. Eur. Polym. J. 2006, 42, 1128–1134. [Google Scholar] [CrossRef]
- Amjadi, M.; Rowshanzamir, S.; Peighambardoust, S.J.; Hosseini, M.G. Investigation of physical properties and cell performance of Nafion/TiO2 nanocomposite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2010, 35, 9252–9260. [Google Scholar] [CrossRef]
- Du, H.; Xu, G.Q.; Chin, W.S.; Huang, L.; Ji, W. Synthesis, Characterization, and Nonlinear Optical Properties of Hybridized CdS−Polystyrene Nanocomposites. ACS Chem. Mater. 2002, 14, 4473–4479. [Google Scholar] [CrossRef]
- Liu, D.; He, G.; Zeng, X.; Sun, D.; Li, X. Preparation of SiO2/epoxy nanocomposite via reverse microemulsion in situ polymerization. Polym. Compos. 2014, 35, 1388–1394. [Google Scholar] [CrossRef]
- Bell, M.; Krentz, T.; Nelson, J.K.; Schadler, L.; Wu, K.; Breneman, C.; Zhao, S.; Hillborg, H.; Benicewicz, B. Investigation of dielectric breakdown in silica-epoxy nanocomposites using designed interfaces. J. Colloid Interface Sci. 2017, 495, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Calebrese, C.; Hui, L.; Schadler, L.S.; Nelson, J.K. A Review on the Importance of Nanocomposite Processing to Enhance Electrical Insulation. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 938–945. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and Mechanical Properties of Polydimethylsiloxane (PDMS)-CaO-SiO2 Hybrids with Different PDMS Contents. J. Sol-Gel Sci. Technol. 2001, 21, 75–81. [Google Scholar] [CrossRef]
- Chen, Q.; Miyaji, F.; Kokubo, T.; Nakamura, T. Apatite formation on PDMS-modified CaO-SiO2-TiO2 hybrids prepared by sol-gel process. Biomaterials 1999, 20, 1127–1132. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Basak, P.; Manorama, S.V. Polymer nanocomposites as solid electrolytes: Evaluating ion-polymer and polymer-nanoparticle interactions in PEG-PU/PAN Semi-IPNs and titania systems. J. Phys. Chem. C 2010, 114, 14281–14289. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; Sengupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Yeung, C.; Vaughan, A.S. On the Effect of Nanoparticle Surface Chemistry on the Electrical Characteristics of Epoxy-Based Nanocomposites. Polymers 2016, 8, 126. [Google Scholar] [CrossRef]
- Virtanen, S.; Krentz, T.; Nelson, J.K.; Schadler, L.; Bell, M.; Benicewicz, B.; Hillborg, H.; Zhao, S. Dielectric Breakdown Strength of Epoxy Bimodal-polymer-Brush-Grafted Core Functionalized Silica Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21. [Google Scholar] [CrossRef]
- Liang, M.; Wong, K.L. Electrical performance of epoxy resin filled with micro particles and nanoparticles. Energy Procedia 2017, 110, 162–167. [Google Scholar] [CrossRef]
- Goyat, M.S.; Rana, S.; Halder, S.; Ghosh, P.K. Facile fabrication of epoxy-TiO2 nanocomposites: A critical analysis of TiO2 impact on mechanical properties and toughening mechanisms. Ultrason. Sonochem. 2018, 40, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.L.; Pearson, R.A. Toughening mechanisms in epoxy–silica nanocomposites (ESNs). Polymer 2009, 50, 4895–4905. [Google Scholar] [CrossRef]
- Roy, M.; Nelson, J.K.; MacCrone, R.K.; Schadler, L.S.; Reed, C.W.; Keefe, R.; Zenger, W. Polymer nanocomposite dielectrics—The role of the interface. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 629–642. [Google Scholar] [CrossRef]
- Roy, M.; Nelson, J.K.; MacCrone, R.K.; Schadler, L.S. Candidate mechanisms controlling the electrical characteristics of silica/XLPE nanodielectrics. J. Mater. Sci. 2007, 42, 3789–3799. [Google Scholar] [CrossRef]
- Ishimoto, K.; Kanegae, E.; Ohki, Y.; Tanaka, T.; Sekiguchi, Y.; Murata, Y.; Reddy, C. Superiority of dielectric properties of LDPE/MgO nanocomposites over microcomposites. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1735–1742. [Google Scholar] [CrossRef]
- Murakami, Y.; Nemoto, M.; Okuzumi, S.; Masuda, S.; Nagao, M. DC Conduction and Electrical Breakdown of MgO/LDPE Nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 290–293. [Google Scholar] [CrossRef]
- Fleming, R.; Ammala, A.; Casey, P.S.; Lang, S.B. Conductivity and space charge in LDPE containing nano-and micro-sized ZnO particles. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 118–126. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer Nanocomposites as Dielectrics and Electrical Insulation-perspectives for Processing Technologies, Material Characterization and Future Applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; et al. Function and characterization of metal oxide-nafion composite membranes for elevated-temperature H2/O2 PEM fuel cells. Chem. Mater. 2006, 18, 2238–2248. [Google Scholar] [CrossRef]
- Shao, Z.-G.; Joghee, P.; Hsing, I.-M. Preparation and characterization of hybrid Nafion-silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Memb. Sci. 2004, 229, 43–51. [Google Scholar] [CrossRef]
- Panero, S.; Fiorenza, P.; Navarra, M.A.; Romanowska, J.; Scrosati, B. Silica-Added, Composite Poly(vinyl alcohol) Membranes for Fuel Cell Application. J. Electrochem. Soc. 2005, 152, A2400. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Rangarajan, R. Organic-Inorganic Hybrid Membrane: Thermally Stable Cation-Exchange Membrane Prepared by the Sol-Gel Method. Macromolecules 2004, 37, 10023–10030. [Google Scholar] [CrossRef]
- Shen, J.; Xi, J.; Zhu, W.; Chen, L.; Qiu, X. A nanocomposite proton exchange membrane based on PVDF, poly(2-acrylamido-2-methyl propylene sulfonic acid), and nano-Al2O3 for direct methanol fuel cells. J. Power Sources 2006, 159, 894–899. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wu, C.-S.; Chiu, Y.-S.; Ho, W.-H. Preparation, Thermal Properties, and Flame Retardance of Epoxy–Silica Hybrid Resins. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2354–2367. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Habouti, S.; Kunstmann-Olsen, C.; Hoyland, J.D.; Rubahn, H.G.; Es-Souni, M. In situ ZnO-PVA nanocomposite coated microfluidic chips for biosensing. Appl. Phys. A Mater. Sci. Process. 2014, 115, 645–649. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Preparation and characterization of a novel conducting nanocomposite blended with epoxy coating for antifouling and antibacterial applications. J. Coat. Technol. Res. 2013, 10, 679–694. [Google Scholar] [CrossRef]
















| Polymer System | Inorganic Component | Inorganic Precursor | Surface Modification | Solvent | Reference |
|---|---|---|---|---|---|
| Epoxy 1 | SiO2 | TEOS | - | - | [54] |
| - | Isopropanol | [55,56,57] | |||
| APTES | Ethanol | [38] | |||
| - | [52] | ||||
| GPTMS | - | [51] | |||
| IPTES | - | [48] | |||
| Ethanol | [49] | ||||
| TEOS, DPTEOS 2 | - | [58] | |||
| APTES | APTES | DMF | [36] | ||
| SiO2, TiO2 | TEOS, TEOT 3 | GPTMS | Acetylacetone | [59] | |
| TiO2 | TIP | - | [60] | ||
| TBO 4 | TCTMTEA 5 | Anhydrous THF | [61] | ||
| SiO2 | TEOS | - | Ionic liquids 6 | [62,63,64] | |
| PDMS | SiO2, TiO2 | TEOS, TIP | - | THF and isopropanol | [65] |
| TiO2 | TIP | - | Ethanol | [66] | |
| - | Isopropanol | [14,67] | |||
| SiO2, TiO2 | TIP, TEOS, MTES 7 | - | [68] | ||
| MxOy 8 | M(OR)n 8 | - | Ethanol | [69,70,71] | |
| ZrO2, TaO2 | ZBO, TE 9 | - | 2-ethoxyethanol | [72] | |
| SiO2–TiO2/ZrO2 | TEOS, TIP, ZP 10 | - | Isopropanol | [73] | |
| MxOy 11 | M(OR)n 11 | - | Ethanol and isopropanol | [74] | |
| SiO2 | TEOS | - | - | [5,75,76,77,78,79,80] | |
| DMDEOS 12 | - | [77] |
| Polymer | Inorganic Component | Inorganic Precursor | Method | Reference |
|---|---|---|---|---|
| Poly(ethylene oxide) | SiO2 | TEOS | Sol–gel | [88] |
| Polypropylene | TiO2 | TBO | [89,90] | |
| SiO2 | TEOS | [91] | ||
| Polyimide | SiO2 | [92] | ||
| Polysulfone | TiO2 | TBO | [101] | |
| Poly(vinyl-co-acetate) | SiO2 | TPO 1 | [102] | |
| Polyethylene-octene | SiO2 | Si(OH)4 | [103] | |
| Polyaniline | SiO2 | TEOS | Combination of sol–gel and in situ polymerization | [93] |
| Poly(ethylene terephthalate) | ZnO | ZA 2 | Combination of sol–gel and electrospinning | [94] |
| SiO2 | TEOS | Sol–gel | [104] | |
| Nafion | TiO2 | TBO | [105] | |
| Unidentified 3 | Si | SiCl4 | Solution-based reduction | [95] |
| Polystyrene | CdS | CA 4 | In situ precipitation | [106] |
| Epoxy | TiO2 | TBO | Solvothermal synthesis | [31] |
| SiO2 | TEOS | Reverse microemulsion | [107] |
| Application | Nanocomposite System | Features | Reference |
|---|---|---|---|
| Electrical insulation | Epoxy–SiO2 Epoxy–TiO2 | Increased dielectric breakdown strength Decreased complex permittivity Increased Tg Increased strength, toughness, ductility | [16,18,108,116,117,118,119,120] |
| PE 1–MgO PE–Al2O3 | Decreased DC conductivity Increased dielectric breakdown strength Decreased space charge accumulation Decreased complex permittivity | [121,122,123,124,125] | |
| PI 2–SiO2 | Decreased electrical conductivity Increased scratch hardness Increased strength | [126] | |
| Fuel cells | Nafion–TiO2 Nafion–SiO2 | Increased water uptake Decreased resistivity Increased Tg Increased degradation temperature | [105,127,128] |
| PVA 3–SiO2 | Increased liquid retention Increased proton conductivity Higher ion-exchange capacity | [129,130] | |
| PVDF 4–Al2O3 | High proton conductivity High thermal stability Low methanol permeability Increased water uptake | [131] | |
| Coatings | Epoxy–SiO2 | Increased flame retardance High Tg Good thermal stability | [132] |
| PDMS–TiO2–SiO2 | Transparent and hydrophobic Increased hardness | [68] | |
| Epoxy–SiO2 Epoxy–Fe2O3 | Improved corrosion resistance Increased Young’s modulus | [133] | |
| PDMS–PVA–ZnO | Decreased hydrophobicity Reduced contamination by fluorescent biomarkers in biosensors | [134] | |
| Epoxy–PANI 5–ZnO | Antifouling and antibacterial properties | [135] | |
| Bioactive materials | PDMS–CaO–SiO2–TiO2 | Increased Young’s modulus High apatite-forming ability High extensibility High strength | [33,111] |
| PDMS–CaO–SiO2 | High apatite-forming ability Decreased release of silicon in body fluids Mechanical properties analogous to those of human cancellous bones | [110] | |
| Solid electrolytes | PEO 6–SiO2 | Increased Li+ transference number Increased Tg | [88] |
| PEG–PU/PAN 7 with TiO2 | Good thermal stability Increased ionic conductivity | [114] | |
| Ultrafiltration | PS8–TiO2 | Increased hydrophilicity Increased permeability Increased Tg | [101] |
| Electromagnetic interference shielding (EMI) | PANI–SiO2 | Increased EMI shielding effectiveness Increased thermal stability | [93] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. https://doi.org/10.3390/polym10101129
Adnan MM, Dalod ARM, Balci MH, Glaum J, Einarsrud M-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers. 2018; 10(10):1129. https://doi.org/10.3390/polym10101129
Chicago/Turabian StyleAdnan, Mohammed M., Antoine R. M. Dalod, Mustafa H. Balci, Julia Glaum, and Mari-Ann Einarsrud. 2018. "In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites" Polymers 10, no. 10: 1129. https://doi.org/10.3390/polym10101129





