Comparative Studies on Phenolic Composition, Antioxidant, Wound Healing and Cytotoxic Activities of Selected Achillea L. Species Growing in Turkey
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Quantification of Phenolic Compounds
| No | Analyte | Parent Ion (m/z) a | MS2 (CE) b | RT c | R2 d | RSD% e | Linearity Range (mg/L) | LOD/LOQ (µg/L) f | Recovery (%) | Quantification (µg Analyte/g Extract) g | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | AK | AL | ||||||||||
| 1 | Quinic acid | 190.95 | 85 (22), 93 (22) | 3.32 | 0.9927 | 0.0388 | 250–10,000 | 22.3/74.5 | 103.3 | 596.6 ± 28.6 | 2545.6 ± 122 | 16214.8 ± 778.3 |
| 2 | Malic acid | 133.05 | 115 (14), 71 (17) | 3.54 | 0.9975 | 0.1214 | 250–10,000 | 19.2/64.1 | 101.4 | 127.6 ± 6.8 | 80.5 ± 4.3 | 40.7 ± 2.2 |
| 3 | tr-Aconitic acid | 172.85 | 85 (12), 129 (9) | 4.13 | 0.9933 | 0.3908 | 250–10,000 | 15.6/51.9 | 102.8 | N.D. h | N.D. | N.D. |
| 4 | Gallic acid | 169.05 | 125 (14), 79 (25) | 4.29 | 0.9901 | 0.4734 | 25–1000 | 4.8/15.9 | 102.3 | N.D. | N.D. | N.D. |
| 5 | Chlorogenic acid | 353 | 191 (17) | 5.43 | 0.9932 | 0.1882 | 250–10,000 | 7.3/24.3 | 99.7 | 511.9 ± 25.1 | 2890.6 ± 141.6 | 778.0 ± 38.1 |
| 6 | Protocatechuic acid | 152.95 | 109 (16), 108 (26) | 5.63 | 0.9991 | 0.5958 | 100–4000 | 25.8/85.9 | 100.2 | N.D. | 53.7 ± 2.7 | 32.8±1.7 |
| 7 | Tannic acid | 182.95 | 124 (22), 78 (34) | 6.46 | 0.9955 | 0.9075 | 100–4000 | 10.2/34.2 | 97.8 | N.D. | N.D. | N.D. |
| 8 | tr-Caffeic acid | 178.95 | 135 (15), 134 (24), 89 (31) | 7.37 | 0.9942 | 1.0080 | 25–1000 | 4.4/14.7 | 98.6 | 9.5 ± 0.5 | 34.9 ± 1.8 | 12.9 ± 0.7 |
| 9 | Vanillin | 151.05 | 136 (17), 92 (21) | 8.77 | 0.9995 | 0.4094 | 250–10,000 | 10.1/33.7 | 99.2 | 40.5 ± 2.0 | 66.0 ± 3.2 | 75.5 ± 3.7 |
| 10 | p-Coumaric acid | 162.95 | 119 (15), 93 (31) | 9.53 | 0.9909 | 1.1358 | 100–4000 | 15.2/50.8 | 98.4 | N.D. | 13.6 ± 0.7 | N.D. |
| 11 | Rosmarinic acid | 358.9 | 161 (17), 133 (42) | 9.57 | 0.9992 | 0.5220 | 250–10,000 | 10.4/34.8 | 101.7 | N.D. | N.D. | N.D. |
| 12 | Rutin | 609.1 | 300 (37), 271 (51), 301 (38) | 10.18 | 0.9971 | 0.8146 | 250–10,000 | 17.0/56.6 | 102.2 | N.D. | 188.1 ± 9.4 | 442.2 ± 22.1 |
| 13 | Hesperidin | 611.1 | 303 (24), 465 (12) | 9.69 | 0.9973 | 0.1363 | 250–10,000 | 21.6/71.9 | 100.2 | N.D. | 422.5 ± 20.7 | 1267.3 ± 62.1 |
| 14 | Hyperoside | 463.1 | 300 (27), 301 (26) | 10.43 | 0.9949 | 0.2135 | 100–4000 | 12.4/41.4 | 98.5 | N.D. | 987.3 ± 48.4 | 167.3 ± 8.2 |
| 15 | 4-OH Benzoic acid | 136.95 | 93 (17), 65 (27) | 11.72 | 0.9925 | 1.4013 | 25–1000 | 3.0/10.0 | 106.2 | 4.2 ± 0.2 | 21.2 ± 1.1 | 22.0 ± 1.1 |
| 16 | Salicylic acid | 136.95 | 93 (16), 65 (31), 75 (30) | 11.72 | 0.9904 | 0.6619 | 25–1000 | 4/13.3 | 106.2 | 10.1 ± 0.5 | 32.9 ± 1.6 | 34.2 ± 1.7 |
| 17 | Myricetin | 317 | 179 (19), 151(23), 137 (26) | 11.94 | 0.9991 | 2.8247 | 100–4000 | 9.9/32.9 | 106.0 | N.D. | N.D. | N.D. |
| 18 | Fisetin | 284.95 | 135 (22), 121 (27) | 12.61 | 0.9988 | 2.4262 | 100–4000 | 10.7/35.6 | 96.9 | N.D. | N.D. | 23.2 ± 1.3 |
| 19 | Coumarin | 146.95 | 103 (17), 91 (26), 77 (27) | 12.52 | 0.9924 | 0.4203 | 100–4000 | 9.1/30.4 | 104.4 | N.D. | N.D. | N.D. |
| 20 | Quercetin | 300.9 | 179 (19), 151 (21), 121 (28) | 14.48 | 0.9995 | 4.3149 | 25–1000 | 2.0/6.8 | 98.9 | N.D. | 28.5 ± 2.0 | 27.8 ± 2.0 |
| 21 | Naringenin | 270.95 | 151 (18), 119 (24), 107 (26) | 14.66 | 0.9956 | 2.0200 | 25–1000 | 2.6/8.8 | 97.0 | 2.6 ± 0.1 | 22.3 ± 1.2 | 4.3 ± 0.2 |
| 22 | Hesperetin | 300.95 | 164 (25), 136 (33), 108 (42) | 15.29 | 0.9961 | 1.0164 | 25–1000 | 3.3/11.0 | 102.4 | N.D. | N.D. | N.D. |
| 23 | Luteolin | 284.95 | 217 (25), 199 (28), 175 (29) | 15.43 | 0.9992 | 3.9487 | 25–1000 | 5.8/19.4 | 105.4 | 29.8 ± 2.1 | 121.2 ± 8.4 | 31.5 ± 2.2 |
| 24 | Kaempferol | 284.95 | 217 (29), 133 (32), 151 (23) | 15.43 | 0.9917 | 0.5885 | 25–1000 | 2.0/6.6 | 99.1 | 45.0 ± 2.3 | 159.4 ± 8.3 | 50.0 ± 2.6 |
| 25 | Apigenin | 268.95 | 151 (25), 117 (35) | 17.31 | 0.9954 | 0.6782 | 25–1000 | 0.1/0.3 | 98.9 | 10.8 ± 0.6 | 797.0 ± 39.9 | 28.5 ± 1.51 |
| 26 | Rhamnetin | 314.95 | 165 (23), 121 (28), 300 (22) | 18.94 | 0.9994 | 2.5678 | 25–1000 | 0.2/0.7 | 100.8 | N.D. | N.D. | N.D. |
| 27 | Chrysin | 253 | 143 (29), 119 (32), 107 (26) | 21.18 | 0.9965 | 1.5530 | 25–1000 | 0.05/0.17 | 102.2 | N.D. | N.D. | N.D. |
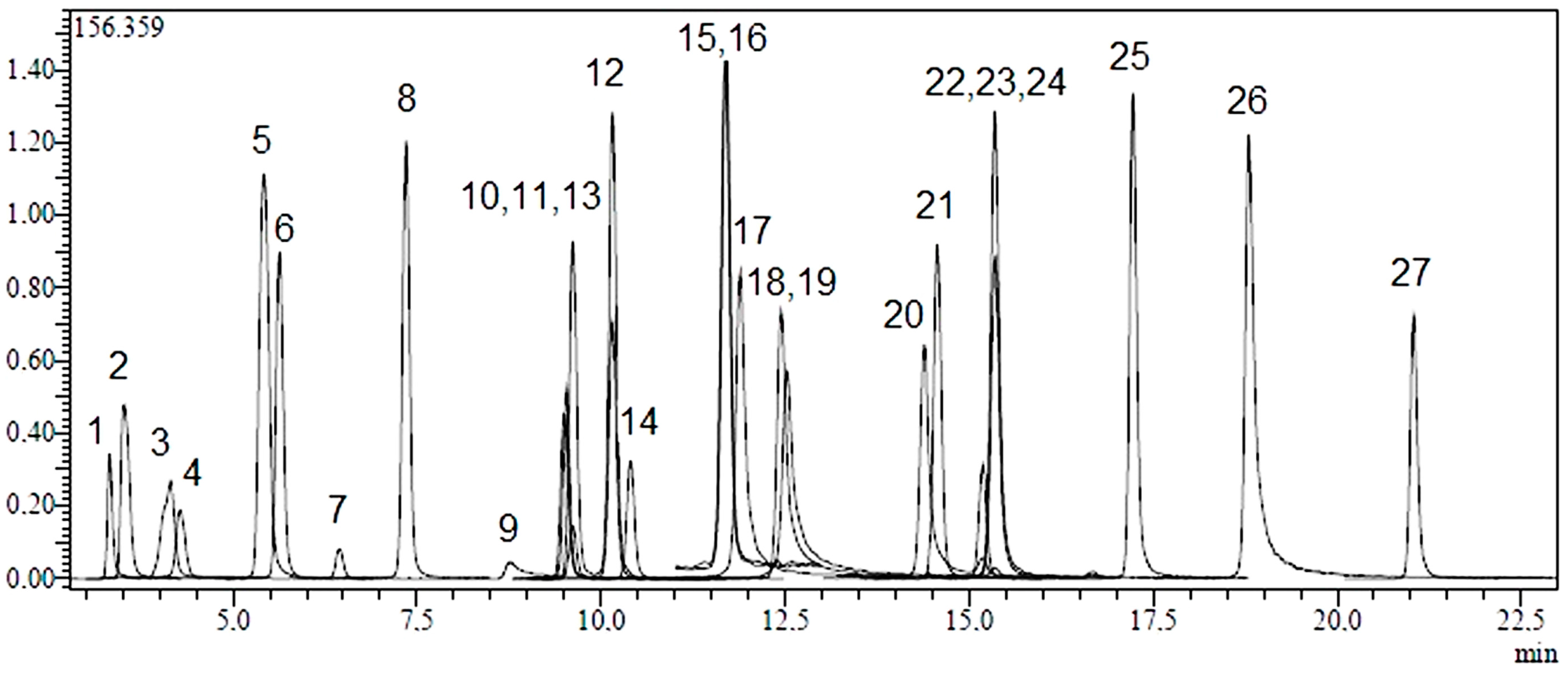
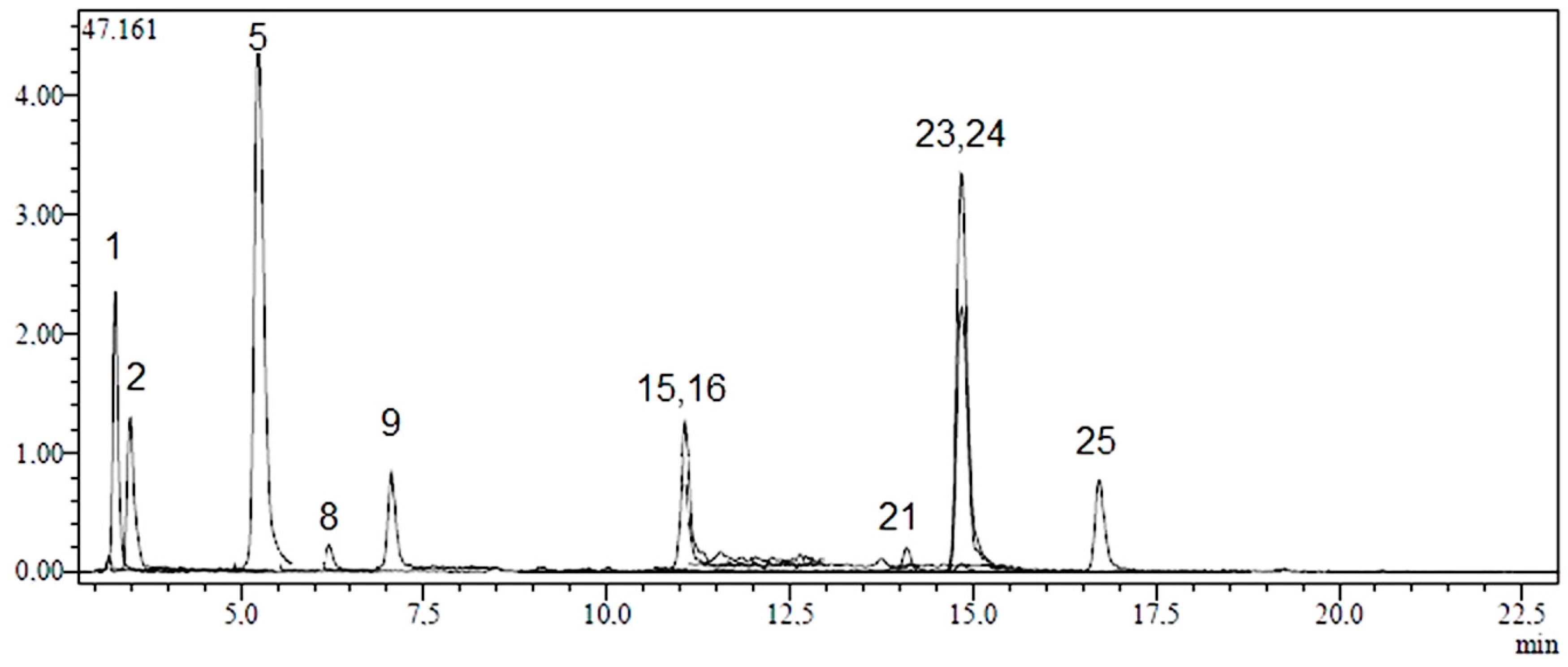
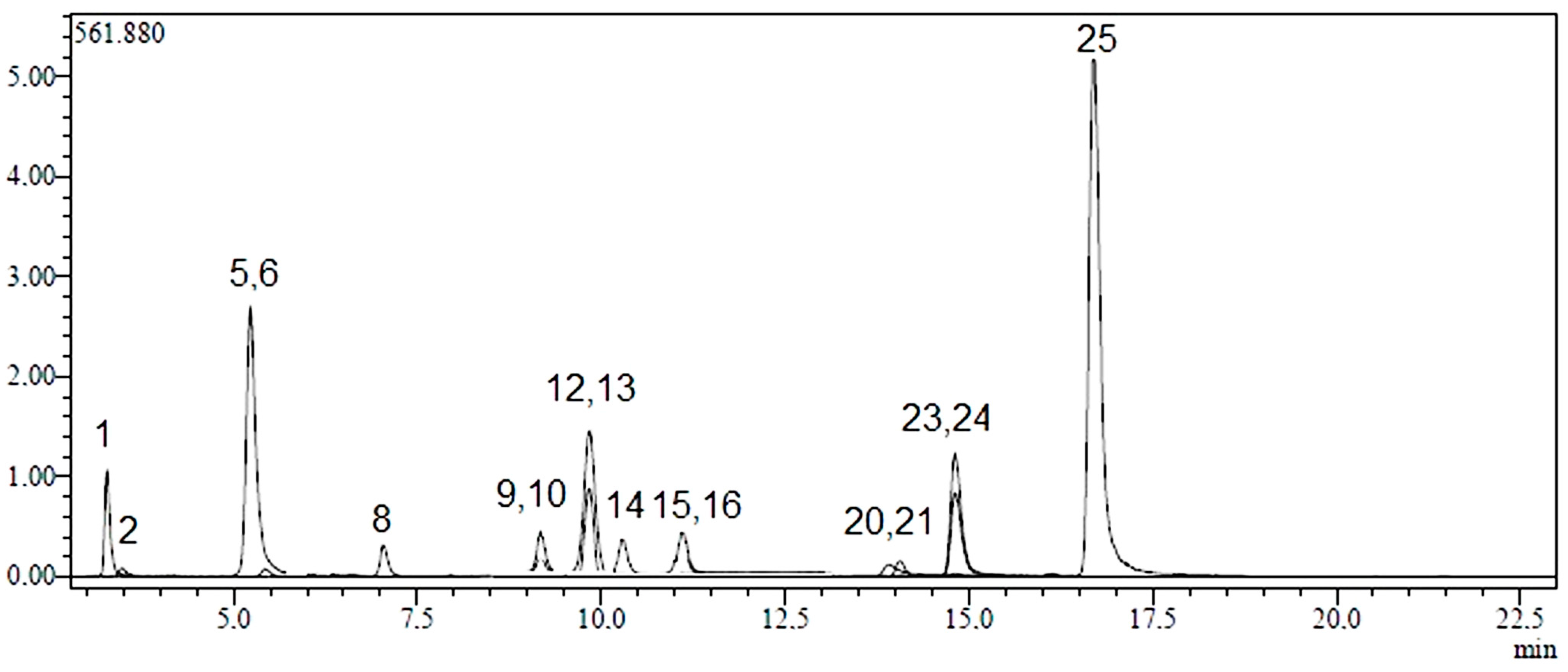
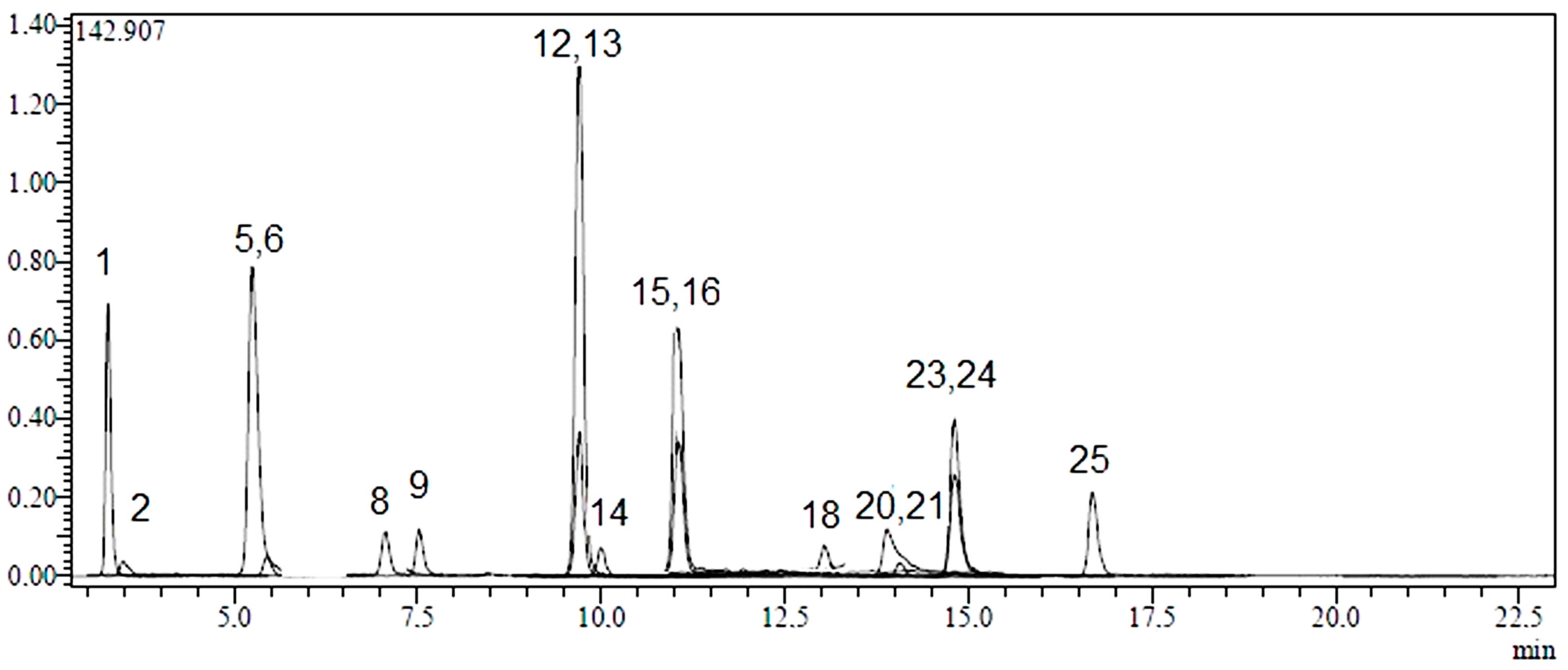
2.2. Total Phenolic Content
| Sample | Total Phenolic Content | DPPH Scavenging Assay | β-Carotene-Linoleic Acid Assay | Total Antioxidant Capacity | |
|---|---|---|---|---|---|
| mg GAE/g Extract 1 | EC50 (μg/mL) | AA (%) 2 | UAE 3 (mM) | CRE 4 (μM) | |
| AC | 55.16 ± 0.96 a | 94.09 ± 3.08c | 74.12 ± 2.11 b | 1.038 ± 0.011a | 2272.91 ± 25.18 a |
| AK | 148.00 ± 0.92 c | 32.63 ± 0.65 a | 60.06 ± 1.39a | 2.080 ± 0.064 b | 4553.85 ± 140.37 b |
| AL | 76.49 ± 1.67 b | 70.01 ± 0.97 b | 81.46 ± 1.65 c | 1.250 ± 0.169 a | 2743.55 ± 369.59 a |
| BHT | - | 29.98 ± 1.15 a | 89.77 ± 0.99 d | - | - |
2.3. Antioxidant Activity
2.3.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.3.2. β-Carotene-Linoleic Acid Assay
2.3.3. Total Antioxidant Capacity (TAC) Assay
2.4. Correlation of Total Phenolic Content and Antioxidant Activity
2.5. Effects on NIH-3T3 Mouse Fibroblast Cells
2.5.1. Cell Proliferation
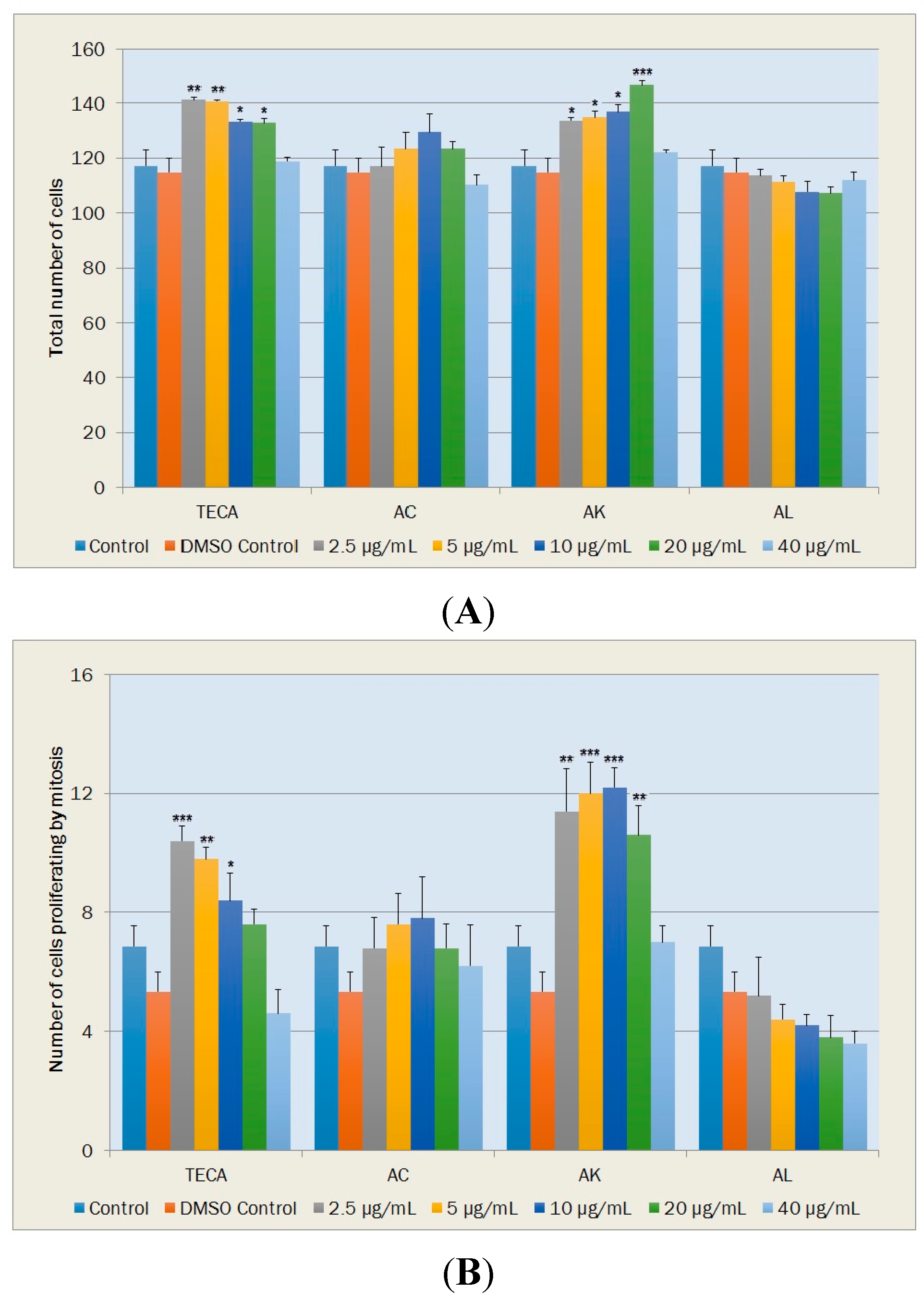
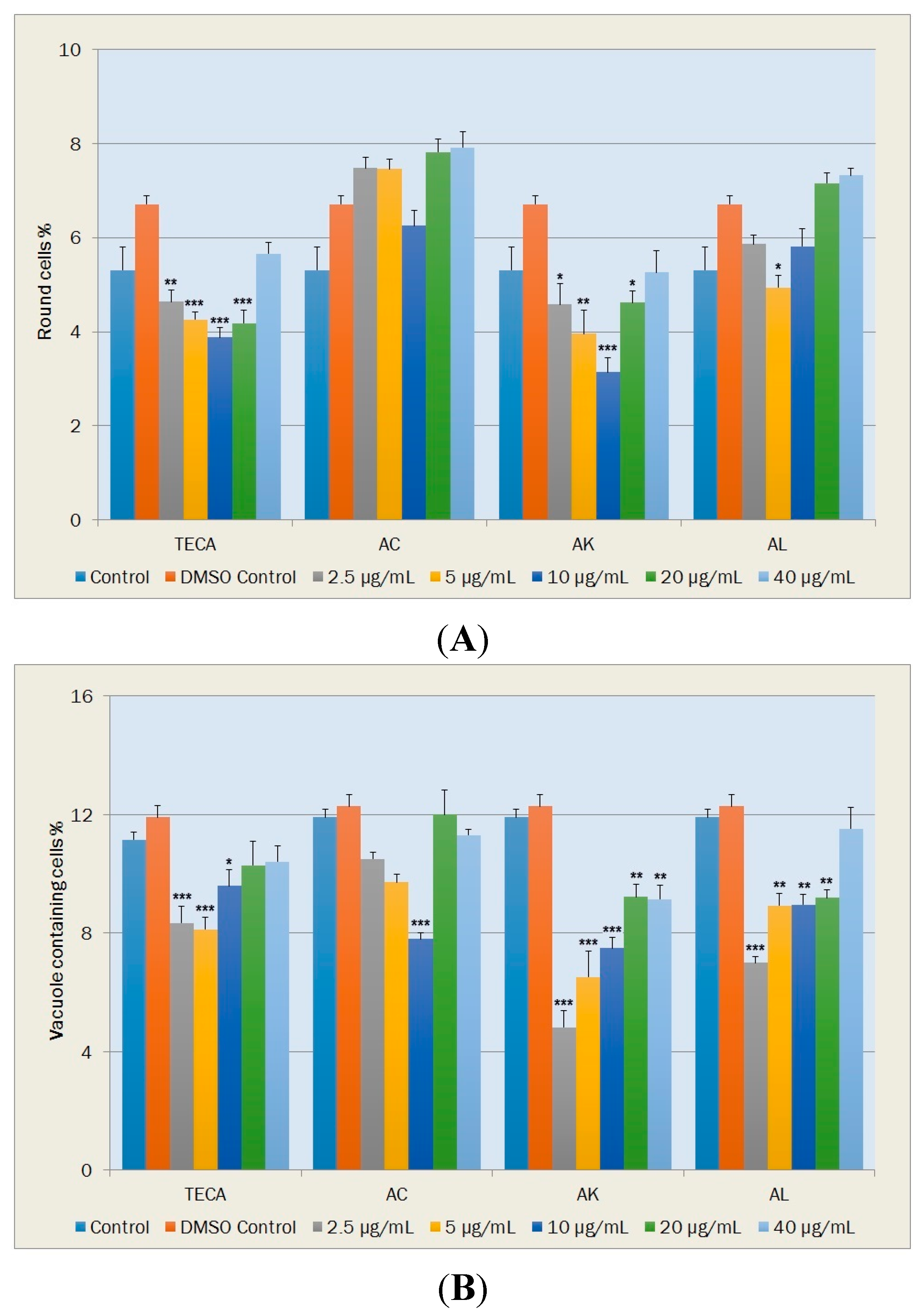
2.5.2. Morphological Changes and Vacuole Containing Cells

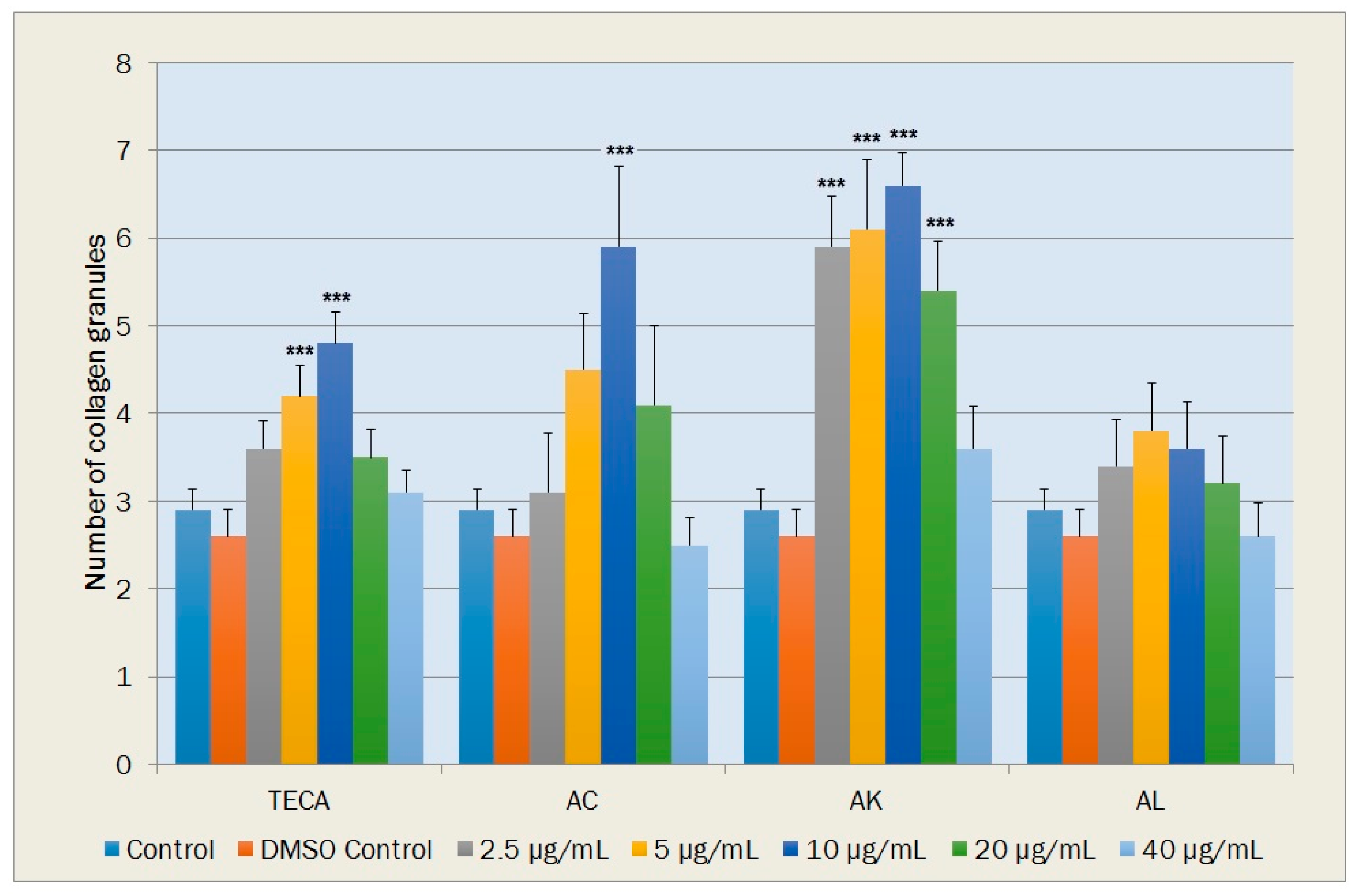
2.5.3. Collagen Production in the Fibroblasts
2.6. Cytotoxic Activity

3. Experimental Section
3.1. Plant Materials
3.2. Extraction Procedure
3.3. Identification and Qualification of Phenolic Compounds by LC-MS/MS
3.4. Determination of Total Phenolic Content
3.5. Antioxidant Activity
3.5.1. DPPH Radical Scavenging Assay
3.5.2. β-Carotene-Linoleic Acid Assay
3.5.3. Total Antioxidant Capacity (TAC) Assay
3.6. Determination of Wound Healing Potency
3.6.1. Cell Culture and Treatment
3.6.2. Staining and Morphometric Analysis of Cultured NIH-3T3 Fibroblasts
3.7. Determination of Cytotoxic Activity
3.7.1. Cell Culture and Treatment
3.7.2. Cytotoxicity/Cell Proliferation Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Turkmenoglu, F.P.; Agar, O.T.; Akaydin, G.; Hayran, M.; Demirci, B. Characterization of the volatile compounds of eleven Achillea species essential oils from Turkey and biological activities of the essential oil and methanol extract of A. hamzaogluii Arabacı & Budak. Molecules 2015, 20, 11432–11458. [Google Scholar] [PubMed]
- Karabay-Yavasoglu, N.U.; Karamenderes, C.; Baykan, S.; Apaydin, S. Antinociceptive and anti-inflammatory activities and acute toxicity of Achillea nobilis subsp. neilreichii extract in mice and rats. Pharm. Biol. 2007, 45, 162–168. [Google Scholar]
- Al-Hindawi, M.K.; Al-Deen, I.H.S.; Nabi, M.H.A.; Ismail, M.A. Anti-inflammatory activity of some Iraqi plants using intact rats. J. Ethnopharmacol. 1989, 26, 163–168. [Google Scholar] [CrossRef]
- Ghasemi, P.A.; Koohpayeh, A.; Karimi, I. Effect of natural remedies on dead space wound healing in wistar rats. Pharmacogn. Mag. 2009, 5, 433–436. [Google Scholar]
- Akkol, K.E.; Koca, U.; Pesin, İ.; Yilmazer, D. Evaluation of the wound healing potential of Achillea biebersteinii Afan. (Asteraceae) by in vivo excision and incision models. J. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Temamogullari, F.; Hayat, A.; Baba, F. Effects of Yarrow Extract on Wound Healing in Rabbits. J. Anim. Vet. Adv. 2009, 8, 1204–1206. [Google Scholar]
- Yaeesh, S.; Jamal, Q.; Khan, A.U.; Gilani, A.H. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother. Res. 2006, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Karamenderes, C.; Apaydın, S. Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bässler on the rat isolated duodenum. J. Ethnopharmacol. 2003, 84, 175–179. [Google Scholar]
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical constituents of the plants in the genus Achillea. Chem. Biodivers. 2006, 3, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Gohari, A.R.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Dai, J.; Mumper, J.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.; Montoro, P.; Piacente, S.; Corona, G.; Deiana, M.; Dessì, M.A.; Pizza, C.; Cabras, P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J. Pharm. Biomed. Anal. 2009, 50, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [PubMed]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725–8739. [Google Scholar] [CrossRef]
- Bobis, O.; Dezmirean, D.S.; Tomos, L.; Chirila, F.; Al. Marghitas, L. Influence of phytochemical profile on antibacterial activity of different medicinal plants against gram-positive and gram-negative bacteria. Appl. Biochem. Microbiol. 2015, 51, 113–118. [Google Scholar] [CrossRef]
- Djurdjević, L.; Gajić, G.; Jarić, S.; Kostić, O.; Mitrović, M.; Pavlović, P. Analysis of benzoic and cinnamic acid derivatives of some medicinal plants in Serbia. Arch. Biol. Sci. Belgrade 2013, 65, 603–609. [Google Scholar] [CrossRef]
- Giorgi, A.; Madeo, M.; Speranza, G.; Cocucci, M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2010, 24, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogoșan, C.; Mocan, A. Comparative Studies on Polyphenolic Composition, Antioxidant and Diuretic Effects of Nigella sativa L. (Black Cumin) and Nigella damascena L. (Lady-in-a-Mist) Seeds. Molecules 2015, 20, 9560–9574. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant Capacities and Analysis of Phenolic Compounds in Three Endemic Nolana Species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta 2014, 25, 332–347. [Google Scholar] [CrossRef]
- Wang, J.C.; Bennett, M. Aging and atherosclerosis mechanisms, functional consequences, and potential therapeutics forcellular senescence. Circ. Res. 2012, 111, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Hylands, P.J.; Mensah, A.Y.; Hensel, A.; Deters, A.M. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Shetty, B.S. Wound healing and indigenous drugs: Role as antioxidants: A Review. Res. Rev. J. Med. Health Sci. 2013, 2, 5–16. [Google Scholar]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crișan, G.; Vlase, L.; Crișan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Baytop, T. Therapy with Medicinal Plants in Turkey, 2nd ed.; Nobel Tıp Publications: Istanbul, Turkey, 1984; pp. 176–177. [Google Scholar]
- Ezer, N.; Mumcu Arisan, Ö. Folk medicines in Merzifon (Amasya, Turkey). Turk. J. Bot. 2006, 30, 223–230. [Google Scholar]
- Kültür, Ş. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Fact Sheet N° 297. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 24 May 2015).
- Ertas, A.; Yilmaz, M.A.; Firat, M. Chemical profile by LC-MS/MS, GC/MS and antioxidant activities of the essential oils and crude extracts of two Euphorbia species. Nat. Prod. Res. 2015, 29, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ertas, A.; Boga, M.; Yilmaz, M.A.; Yesil, Y.; Tel, G.; Temel, H.; Hasimi, N.; Gazioglu, I.; Ozturk, M.; Ugurlu, P. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): Rosmarinic acid. Ind. Crop. Prod. 2015, 67, 336–345. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin—Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Konyalıoğlu, S.; Karamenderes, C. Screening of total phenol contents ans antioxidant capacities of some Achillea L. species growing in Turkey. Acta Pharm. Turc. 2004, 46, 163–170. [Google Scholar]
- Bashi, D.S.; Fazly Bazzaz, B.S.; Sahebkar, A.; Karimkhani, M.M.; Ahmadi, A. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm. Biol. 2012, 50, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Baris, D.; Kizil, M.; Aytekin, C.; Kizil, G.; Yavuz, M.; Ceken, B.; Ertekin, S. The in Vitro Antimicrobial and Antioxidant Activity of Ethanol Extract of Three Hypericum and Three Achillea Species from Turkey. Int. J. Food Prop. 2011, 14, 339–355. [Google Scholar] [CrossRef]
- Giorgi, A.; Bombelli, R.; Luini, A.; Speranza, G.; Cosentino, M.; Lecchini, S.; Cocucci, M. Antioxidant and cytoprotective properties of infusions from leaves and inflorescencesof Achillea collina Becker ex Rchb. Phytother. Res. 2009, 23, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Shahsavari, N.; Barzegar, M.; Sahari, M.A.; Naghdibadi, H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum. Nutr. 2008, 63, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Konyalioglu, S.; Karamenderes, C. The protective effects of Achillea L. species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J. Ethnopharmacol. 2005, 102, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lee, S.M.; Lin, Y.J.; Chiang, S.H.; Lin, C.C. Effects of Danshensu and Salvianolic Acid B from Salvia miltiorrhiza Bunge (Lamiaceae) on cell proliferation and collagen and melanin production. Molecules 2014, 19, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.F.; Diegelmann, R.F.; Cohen, I.K. An in vitro model of fibroplasia: Simultaneous quantification of fibroblast proliferation, migration, and collagen synthesis. Proc. Soc. Exp. Biol. Med. 1984, 176, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures bytriterpene extracted from Centella asiatica. Connect. Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Zeytinoglu, H.; Zeytinoglu, M.; Aydın, S.; Öztürk, Y.; Baser, K.H.C. Testing the wound-healing activity in T15 fibroblast culture: A morphometric analysis. Altern. Lab. Anim. 2000, 28, 41–51. [Google Scholar] [PubMed]
- Öztürk, N.; Korkmaz, S.; Öztürk, Y. Wound-healing activity of St. John’s Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J. Ethnopharmacol. 2007, 111, 33–39. [Google Scholar] [CrossRef]
- Dikmen, M.; Öztürk, Y.; Sagratini, G.; Ricciutelli, M.; Vittori, S.; Maggi, F. Evaluation of the wound healing potentials of two subspecies of Hypericum perforatum on cultured NIH3T3 fibroblasts. Phytother. Res. 2011, 25, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, G.; Sardari, S.; Shokrgozar, M.A. Anticancerous potentials of Achillea species against selected cell lines. J. Med. Plants Res. 2010, 4, 2411–2417. [Google Scholar]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative Effect of Flavonoids and Sesquiterpenoids from Achillea millefolium s.l. on Cultured Human Tumour Cell Lines. Phytother. Res. 2009, 23, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Küpeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Almeida, J.S.; Benvegnú, D.M.; Boufleur, N.; Reckziegel, P.; Barcelos, R.C.; Coradini, K.; de Carvalho, L.M.; Bürger, M.E.; Beck, R.C. Hydrogels containing rutin intended for cutaneous administration: Efficacy in wound healing in rats. Drug Dev. Ind. Pharm. 2012, 38, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn-Schmiedebergs Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary Metabolites from Plants Inhibiting ABC Transporters and Reversing Resistance of Cancer Cells and Microbes to Cytotoxic and Antimicrobial Agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Beara, I.N.; Lesjak, M.M.; Cetojević-Simin, D.D.; Marjanović, Z.S.; Ristić, J.D.; Mrkonjić, Z.O.; Mimica-Dukić, N.M. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of endemic Plantago reniformis G. Beck. Food Chem. 2014, 165, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ying, M.D.; Wu, Y.P.; Zhou, Z.H.; Ye, Z.M.; Li, H.; Lin, D. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, M.; Canturk, Z.; Ozturk, Y.; Tunali, Y. Investigation of the apoptotic effect of curcumin in human leukemia HL-60 cells by using flow cytometry. Cancer Biother. Radiopharm. 2010, 25, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the plants are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agar, O.T.; Dikmen, M.; Ozturk, N.; Yilmaz, M.A.; Temel, H.; Turkmenoglu, F.P. Comparative Studies on Phenolic Composition, Antioxidant, Wound Healing and Cytotoxic Activities of Selected Achillea L. Species Growing in Turkey. Molecules 2015, 20, 17976-18000. https://doi.org/10.3390/molecules201017976
Agar OT, Dikmen M, Ozturk N, Yilmaz MA, Temel H, Turkmenoglu FP. Comparative Studies on Phenolic Composition, Antioxidant, Wound Healing and Cytotoxic Activities of Selected Achillea L. Species Growing in Turkey. Molecules. 2015; 20(10):17976-18000. https://doi.org/10.3390/molecules201017976
Chicago/Turabian StyleAgar, Osman Tuncay, Miris Dikmen, Nilgun Ozturk, Mustafa Abdullah Yilmaz, Hamdi Temel, and Fatma Pinar Turkmenoglu. 2015. "Comparative Studies on Phenolic Composition, Antioxidant, Wound Healing and Cytotoxic Activities of Selected Achillea L. Species Growing in Turkey" Molecules 20, no. 10: 17976-18000. https://doi.org/10.3390/molecules201017976






