Preparation and Characterization of Natural Bleaching Gels Used in Cosmetic Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Bleaching Gels
2.2. HPLC Analysis of Organic Acid Content
2.3. Experimental Composites for Restoration
2.4. Determining of the Color Changes of the Restoration Materials after Application of the Investigated Bleaching Gels
2.5. Fluorescence Spectroscopic Evaluation of Composites before and after the Bleaching Process
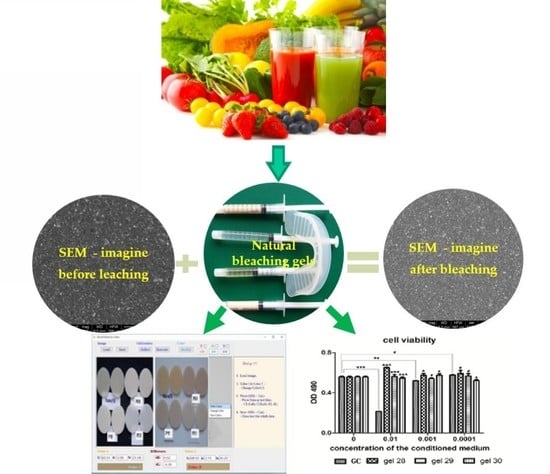
2.6. Microstructural Analysis of Composites by SEM
2.7. Biological Assays
3. Results
3.1. Determination of Organic Acid Content (mg/g) in Bleaching Gels by HPLC Analysis
3.2. Determining the Color Change after Applying the Whitening Gels by Computerized Measurement
3.3. Fluorescence Spectroscopic Evaluation of Composites before and after the Bleaching Process
3.4. Microstructural Analysis of Samples of Composite Materials before, after Staining, and after Bleaching
3.5. Biological Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paravina, R.D.; Roeder, L.; Lu, H.; Vogel, K.; Powers, J.M. Effect of finishing and polishing procedures on surface roughness, gloss and color of resin-based composites. Am. J. Dent. 2004, 17, 262–266. [Google Scholar] [PubMed]
- Greenwall, L. Bleaching Techniques in Restorative Dentistry, 1st ed.; CRC Press Taylor & Francis Group: London, UK, 2001; p. 428. [Google Scholar]
- Watts, A.; Addy, M. Tooth discolouration and staining: Tooth discolouration and staining: A review of the literature. Br. Dent. J. 2001, 190, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.A.; Ellwood, R.; Davies, R. A six-month study of two self-applied tooth whitening products containing carbamide peroxide. Oper. Dent. 2004, 29, 623–626. [Google Scholar] [PubMed]
- Caughman, W.F.; Frazier, K.B.; Haywood, V.B. Carbamide peroxide whitening of nonvital single discolored teeth: case reports. Quintessence Int. 1999, 30, 155–161. [Google Scholar] [PubMed]
- Clark, D.M.; Hintz, J. Case Report: In-Office Tooth Whitening Procedure with 35% Carbamide Peroxide Evaluated by the Minolta CR-321 Chroma Meter. J. Esthet. Restor. Dent. 1998, 10, 37–42. [Google Scholar] [CrossRef]
- Croll, T.P. Tooth bleaching by different concentrations of carbamide peroxide and hydrogen peroxide whitening strips: An in vitro study-commentary. J. Esthet. Restor. Dent. 2006, 18, 101. [Google Scholar] [CrossRef]
- De Vasconcelos, A.; Cunha, A.; Borges, B.; Machado, C.; Dos Santos, A. Tooth whitening with hydrogen/carbamide peroxides in association with a CPP-ACP paste at different proportions. Aust. Dent. J. 2012, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Delgado, E.; Hernández-Cott, P.L.; Stewart, B.; Collins, M.; De Vizio, W. Tooth-whitening efficacy of custom tray-delivered 9% hydrogen peroxide and 20% carbamide peroxide during daytime use: A 14-day clinical trial. Puerto Rico Heal. Sci. J. 2007, 26, 367–372. [Google Scholar]
- Joiner, A. The bleaching of teeth: A review of the literature. J. Dent. 2006, 34, 412–419. [Google Scholar] [CrossRef]
- Strassler, H. The Science and Art Tooth Whitening, Tusla, OK, PenaWellCorp. Available online: https://www.ineedce.com/courses/1698/PDF/TheScienceandArtWhitening.pdf (accessed on 27 February 2019).
- Jeon, J.-G.; Rosalen, P.; Falsetta, M.; Koo, H. Natural Products in Caries Research: Current (Limited) Knowledge, Challenges and Future Perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef]
- Badria, F.A.; Zidan, O.A. Natural Products for Dental Caries Prevention. J. Med. Food 2004, 7, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Palombo, E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evidence Based Complement. Altern. Med. 2011, 2011, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, A.; Polimeni, A.; Strohmenger, L.; Cicciù, D.; Gherlone, E.; Abati, S. Dental patients’ self-reports of xerostomia and associated risk factors. J. Am. Dent. Assoc. 2011, 142, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Schemehorn, B.R.; Luo, D.; E Kohut, B.; Parikh, R.; Santos, S.L. Stain removal efficacy of two new fluoride dentifrices containing essential oils. Am. J. Dent. 2000, 13, 23–25. [Google Scholar]
- Xie, P.; Lu, J.; Wan, H.; Hao, Y. Effect of toothpaste containing d-limonene on natural extrinsic smoking stain: A 4-week clinical trial. Am. J. Dent. 2010, 23, 196–200. [Google Scholar] [PubMed]
- Kalyana, P.; Shashidhar, A.; Meghashyam, B.; Sreevidya, K.R.; Sweta, S. Stain removal efficacy of a novel dentifrice containing papain and Bromelain extracts—An in vitro study. Int. J. Dent. Hyg. 2011, 9, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; Mahdavi, S.; Loyn, T. Dietary staining in vitro by mouthrinses as a comparative measure of antiseptic activity and predictor of staining in vivo. J. Dent. 1995, 23, 95–99. [Google Scholar] [CrossRef]
- Joiner, A.; Muller, D.; Elofsson, U.M.; Malmsten, M.; Arnebrant, T. Adsorption from black tea and red wine onto in vitro salivary pellicles studied by ellipsometry. Eur. J. Oral Sci. 2003, 111, 417–422. [Google Scholar] [CrossRef]
- Aldred, M.J.; Talacko, A.A.; Ruljancich, K.; Story, R.D.; Newland, S.; Chen, S.T.; Bergman, J.D.; Smith, A.; Dimitroulis, G.; Redman, J.; et al. Oral focal mucinosis: report of 15 cases and review of the literature. Pathology 2003, 35, 393–396. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Scully, C.; Bagan-Sebastian, J.-V. Drug-induced Disorders of Teeth. J. Dent. Res. 2005, 84, 596–602. [Google Scholar] [CrossRef]
- Heymann, H.O.; Swift, E.J., Jr.; Bayne, S.C. Clinical evaluation of two carbamide peroxide tooth-whitening agents. Compend. Contin. Educ. Dent. 1998, 19, 359–376. [Google Scholar] [PubMed]
- Kelleher, M.; Roe, F. The safety-in-use of 10% carbamide peroxide (Opalescence) for bleaching teeth under the supervision of a dentist. Br. Dent. J. 1999, 187, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Niederman, R.; Tantraphol, M.C.; Slinin, P.; Hayes, C.; Conway, S. Effectiveness of dentist-prescribed, home-applied tooth whitening. A meta analysis. J. Contemp. Dent. Pr. 2000, 1, 20–36. [Google Scholar]
- Jiang, T.; Ma, X.; Wang, Z.; Tong, H.; Hu, J.; Wang, Y. Beneficial effects of hydroxyapatite on enamel subjected to 30% hydrogen peroxide. J. Dent. 2008, 36, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-H.; An, J.-H.; Kim, B.-K.; Hwang, I.-N.; Park, Y.-J.; Song, H.-J. Effect of laser irradiation on crystalline structure of enamel surface during whitening treatment with hydrogen peroxide. J. Dent. 2012, 40, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.; Moldovan, M.; Vlassa, M.; Sarosi, C.; Cojocaru, I. HPLC Determination of the Main Organic Acids in Teeth Bleaching Gels Prepared with the Natural Fruit Juices. Rev.Chim. 2016, 67, 2440–2445. [Google Scholar]
- Baldea, I.; Olteanu, D.E.; Filip, A.G.; Cenariu, M.; Dudea, D.; Tofan, A.; Alb, C.; Moldovan, M. Toxicity and efficiency study of plant extracts-based bleaching agents. Clin. Oral Investig. 2017, 21, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.N.; Rode, S.M.; Marques, M.M. Cytotoxicity of substances leached or dissolved from pulp capping materials. Int. Endod. J. 2005, 38, 505–509. [Google Scholar] [CrossRef]
- Rodgers, A.L.; Webber, D.; De Charmoy, R.; Jackson, G.E.; Ravenscroft, N. Malic Acid Supplementation Increases Urinary Citrate Excretion and Urinary pH: Implications for the Potential Treatment of Calcium Oxalate Stone Disease. J. Endourol. 2014, 28, 229–236. [Google Scholar] [CrossRef]
- Healthline. Available online: https://www.healthline.com/health/malic-acid-skin-care#skin-care-science (accessed on 14 March 2019).
- Cosmetic Ingredient Review. Available online: https://www.cir-safety.org/sites/default/files/malic%20acid.pdf (accessed on 14 March 2019).
- Prejmerean, V.; Silaghi-Dumitrescu, L.; Prodan, D.; Prejmerean, C.; Moldovan, M. Study of some Dental Biomaterials Properties using an Original Software Application. Key Eng. Mater. 2014, 587, 121–127. [Google Scholar] [CrossRef]
- Mokhlis, G.R.; Matis, B.A.; Cochran, M.A.; Eckert, G.J. A Clinical Evaluation of Carbamide Peroxide and Hydrogen Peroxide Whitening Agents during Daytime Use. J. Am. Dent. Assoc. 2000, 131, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, T.; Takahashi, M.K.; Brum, R.T.; Rached, R.N.; Souza, E.M. Comparative study of the fluorescence intensity of dental composites and human teeth submitted to artificial aging. Gen. Dent. 2014, 62, 37–41. [Google Scholar] [PubMed]
- Wu, C.-J.; Gaharwar, A.K.; Schexnailder, P.J.; Schmidt, G. Development of Biomedical Polymer-Silicate Nanocomposites: A Materials Science Perspective. Materials 2010, 3, 2986–3005. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, M.R. Novel Advances and Approaches in Biomedical Materials Based on Calcium Phosphates. Materials 2019, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Bartek Ingredients. Available online: http://www.bartek.ca/pdfs/Applications/PersonalCareProducts/Malic%20Acid%20in%20Facial%20Skincare%20Preparations.pdf (accessed on 14 March 2019).
- Tavares, C.; Correia, A.; Bresciani, E.; Caneppele, T.; Tavares, L. Effect of thickness on fluorescence of resin-composites and dental substrates. Braz. Dent. Sci. 2016, 19, 53. [Google Scholar] [CrossRef]
- Park, M.Y.; Lee, Y.K.; Lim, B.S. Influence of fluorescent whitening agent on the fluorescent emission of resin composites. Dent. Mater. 2007, 23, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lu, H.; Powers, J.M. Influence of fluorescent and opalescent properties of resin composites on the masking effect. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 26–32. [Google Scholar] [CrossRef]
- Fukushima, Y.; Araki, T.; Yamada, M. Topography of fluorescence and its possible composites in human teeth. Cell. Mol. Boil. 1987, 33, 725–736. [Google Scholar]
- Matsumoto, H.; Kitamura, S.; Araki, T. Autofluorescence in human dentine in relation to age, tooth type and temperature measured by nanosecond time-resolved fluorescence microscopy. Arch. Oral Boil. 1999, 44, 309–318. [Google Scholar] [CrossRef]
- Al-Ameedee, A.H. Physico-Chemical Analysis of Effect of In-Office Tooth Bleaching Agent on Three Esthetic Composite Resin Restorations (in vitro Study). Asian J. Chem. 2017, 29, 763–770. [Google Scholar] [CrossRef]
- Junior, M.T.; Rodrigues, C.A.; Bernardes, V.L.; Araujo, T.S.B.; Nicoli, G.A.; dos Reis Derceli, J. Dental Bleaching and New Possibilities—Literature Review. Health Sci. J. 2018, 12, 1–6. [Google Scholar] [CrossRef]
- Polyzois, G. In vitro evaluation of dental materials. Clin. Mater. 1994, 16, 21–60. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R. Biocompatibility of Resin-based Dental Materials. Materials 2009, 2, 514–548. [Google Scholar] [CrossRef] [Green Version]








| Composite | Organic Matrix | Fillers | Ratio L/F [wt] |
|---|---|---|---|
| P11 * | Bis-GMA*, TEGDMA, UDMA | Glass with BaF2 *, colloidal silica, HA-Zn * | 23/77 |
| P31 * | Bis-GMA*, TEGDMA, UDMA | Glass with BaO *, Mixture of oxides *, Colloidal silica | 23/77 |
| P61 * | Bis-GMA*, TEGDMA, UDMA | Glass with Sr si Zr *, SiO2-Zr *, HA-F *, HA-Zr * | 21.8/78.19 |
| Nanofill | UDMA-resin | 0.1–2 μm glass and 100 nm nanoparticles | 18/82 |
| GEL Code | Oxalic Acid | Citric Acid | Tartaric Acid | Malic Acid | Succinic Acid | Fumaric Acid | Total Acidity |
|---|---|---|---|---|---|---|---|
| GEL 28 | - | 0.132 | 0.124 | 33.39 | 6.216 | 0.0009 | 39.8629 |
| GEL 29 | 0.0165 | 2.608 | - | 14.146 | 2.55 | 0.0089 | 19.3294 |
| GEL 30 | - | 6.77 | - | 9.859 | 3.73 | 0.0066 | 20.3656 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazilu, A.; Sarosi, C.; Moldovan, M.; Miuta, F.; Prodan, D.; Antoniac, A.; Prejmerean, C.; Silaghi Dumitrescu, L.; Popescu, V.; Raiciu, A.D.; et al. Preparation and Characterization of Natural Bleaching Gels Used in Cosmetic Dentistry. Materials 2019, 12, 2106. https://doi.org/10.3390/ma12132106
Mazilu A, Sarosi C, Moldovan M, Miuta F, Prodan D, Antoniac A, Prejmerean C, Silaghi Dumitrescu L, Popescu V, Raiciu AD, et al. Preparation and Characterization of Natural Bleaching Gels Used in Cosmetic Dentistry. Materials. 2019; 12(13):2106. https://doi.org/10.3390/ma12132106
Chicago/Turabian StyleMazilu (Moldovan), Amalia, Codruta Sarosi, Marioara Moldovan, Filip Miuta, Doina Prodan, Aurora Antoniac, Cristina Prejmerean, Laura Silaghi Dumitrescu, Violeta Popescu, Anca Daniela Raiciu, and et al. 2019. "Preparation and Characterization of Natural Bleaching Gels Used in Cosmetic Dentistry" Materials 12, no. 13: 2106. https://doi.org/10.3390/ma12132106