Combination of Berberine with Resveratrol Improves the Lipid-Lowering Efficacy
Abstract
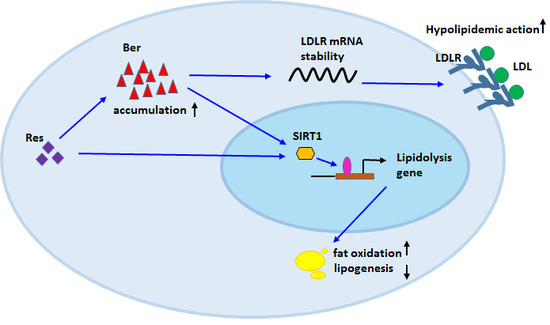
:1. Introduction
2. Results
2.1. The Anti-Hyperlipidemic Effect of Combined Therapy with Berberine and Resveratrol in Hyperlipidemic Mice and 3T3-L1 Adipocytes
2.2. Resveratrol Increased the Accumulation of Berberine in Hepatic L02 Cells
2.3. The Combined Therapy Can Increase Low-Density-Lipoprotein Receptor Expression in Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Study Protocol
4.2. Cell Culture and Adipocytes Differentiation
4.3. Oil Red O Staining Assay
4.4. Western Blot Assay
4.5. Flow Cytometry Assay
4.6. Statistical Analysis.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ber | Berberine |
| Res | Resveratrol |
| SIRT1 | Sirtuin 1 |
| TC | Total cholesterol |
| LDLR | Low Density Lipoprotein Receptor |
| HFD | High fat diet |
| TG | triglyceride |
| LDL-c | Low Density Lipoprotein cholesterol |
| MFI | mean fluorescence intensity |
| FBS | Fetal Bovine Serum |
| DMSO | Dimethyl Sulphoxide |
| PBS | phosphate buffered saline |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| PVDF | Polyvinylidene fluoride |
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- McKeigue, P.M.; Shah, B.; Marmot, M.G. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991, 337, 382–386. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Gong, Z.; Sheng, X.; Li, Z.; Zhang, W.; Qin, Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPAR-gamma pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, X.; Mao, G.; Gao, Z.; Wang, H.; He, Q.; Li, D. Hepatoprotection of berberine against hydrogen peroxide-induced apoptosis by upregulation of Sirtuin 1. Phytother. Res. 2013, 27, 417–421. [Google Scholar] [CrossRef]
- Zhu, X.; Yue, H.; Guo, X.; Yang, J.; Liu, J.; Liu, J.; Wang, R.; Zhu, W. The Preconditioning of Berberine Suppresses Hydrogen Peroxide-Induced Premature Senescence via Regulation of Sirtuin 1. Oxid. Med. Cell Longev. 2017, 2017, 2391820. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Cohen, D.; Robinson, A.; Motta, M.C.; van Veen, E.; Czopik, A.; Steele, A.D.; Crowe, H.; Marmor, S.; Luo, J.; et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007, 6, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 27, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talwalkar, S.S.; Vaidya, A.B.; Godse, C.; Vaidya, A.; Vaidya, R. Plasmodium DNA Fluoresces with berberine: A novel approach for diagnosis of malarial parasites. Am. J. Clin. Pathol. 2005, 124, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.L.; Oliveira, P.J.; Sardao, V.A.; Perkins, E.; Parke, D.; Holy, J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother. Pharmacol. 2008, 61, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural plant chemicals: Sources of industrial and medicinal materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.R.; Oh, G.T.; Park, H.S.; Lee, K.U.; Lane, M.D.; et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.P.; Duarte, F.V.; Nunes, P.; Hubbard, B.P.; Teodoro, J.S.; Varela, A.T.; Jones, J.G.; Sinclair, D.A.; Palmeira, C.M.; Rolo, A.P. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta 2012, 1822, 185–195. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Koneru, S.; Juhasz, B.; Zhan, L.; Pant, R.; Menon, V.P.; Otani, H.; Maulik, N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell. Cardiol. 2007, 42, 508–516. [Google Scholar] [CrossRef]
- Guo, T.; Woo, S.L.; Guo, X.; Li, H.; Zheng, J.; Botchlett, R.; Liu, M.; Pei, Y.; Xu, H.; Cai, Y.; et al. Berberine Ameliorates Hepatic Steatosis and Suppresses Liver and Adipose Tissue Inflammation in Mice with Diet-induced Obesity. Sci. Rep. 2016, 6, 22612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.J.; Zhang, H.; Song, D.Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.M.; Shan, N.; Zhou, Z.X.; Yang, P.; et al. Berberine reduce insulin-resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N.; Tanaka, M.; Terai, K.; Ueda, K.; Yazaki, K. Human MDR1 and MRP1 recognize berberine as their transport substrate. Biosci. Biotechnol. Biochem. 2007, 71, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.; Pan, C.; Ma, Q.; Zhang, S.; Yan, L. Reversal effect of resveratrol on multidrug resistance in KBv200 cell line. Biomed. Pharmacother. 2008, 62, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of chemopreventive citrus phytochemicals on human P-glycoprotein and multidrug resistance protein 1. Eur. J. Pharmacol. 2008, 600, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Kim, E.; Kang, J.; Han, H.; Sul, S.; Park, S.B.; Kim, S.K. Photochemical generation of a new, highly fluorescent compound from non-fluorescent resveratrol. Chem. Commun. 2012, 48, 3839–3841. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, J.L.; Chandrasekaran, C.; Cooper, A.D. Evidence for sterol-independent regulation of low-density lipoprotein receptor activity in Hep-G2 cells. Biochem. J. 1991, 279, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell. Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef]
- Miranda, M.X.; van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; Stein, S.; Gomes, A.P.; Suri, V.; Ellis, J.L.; et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur. Heart J. 2015, 36, 51–59. [Google Scholar] [CrossRef]
- Student, A.K.; Hsu, R.Y.; Lane, M.D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 1980, 255, 4745–4750. [Google Scholar]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Yang, J.; Zhu, W.; Yin, X.; Yang, B.; Wei, Y.; Guo, X. Combination of Berberine with Resveratrol Improves the Lipid-Lowering Efficacy. Int. J. Mol. Sci. 2018, 19, 3903. https://doi.org/10.3390/ijms19123903
Zhu X, Yang J, Zhu W, Yin X, Yang B, Wei Y, Guo X. Combination of Berberine with Resveratrol Improves the Lipid-Lowering Efficacy. International Journal of Molecular Sciences. 2018; 19(12):3903. https://doi.org/10.3390/ijms19123903
Chicago/Turabian StyleZhu, Xiaofei, Jingyi Yang, Wenjuan Zhu, Xiaoxiao Yin, Beibei Yang, Yihui Wei, and Xiaofang Guo. 2018. "Combination of Berberine with Resveratrol Improves the Lipid-Lowering Efficacy" International Journal of Molecular Sciences 19, no. 12: 3903. https://doi.org/10.3390/ijms19123903