Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Composition of Raw and Aged Black Garlic
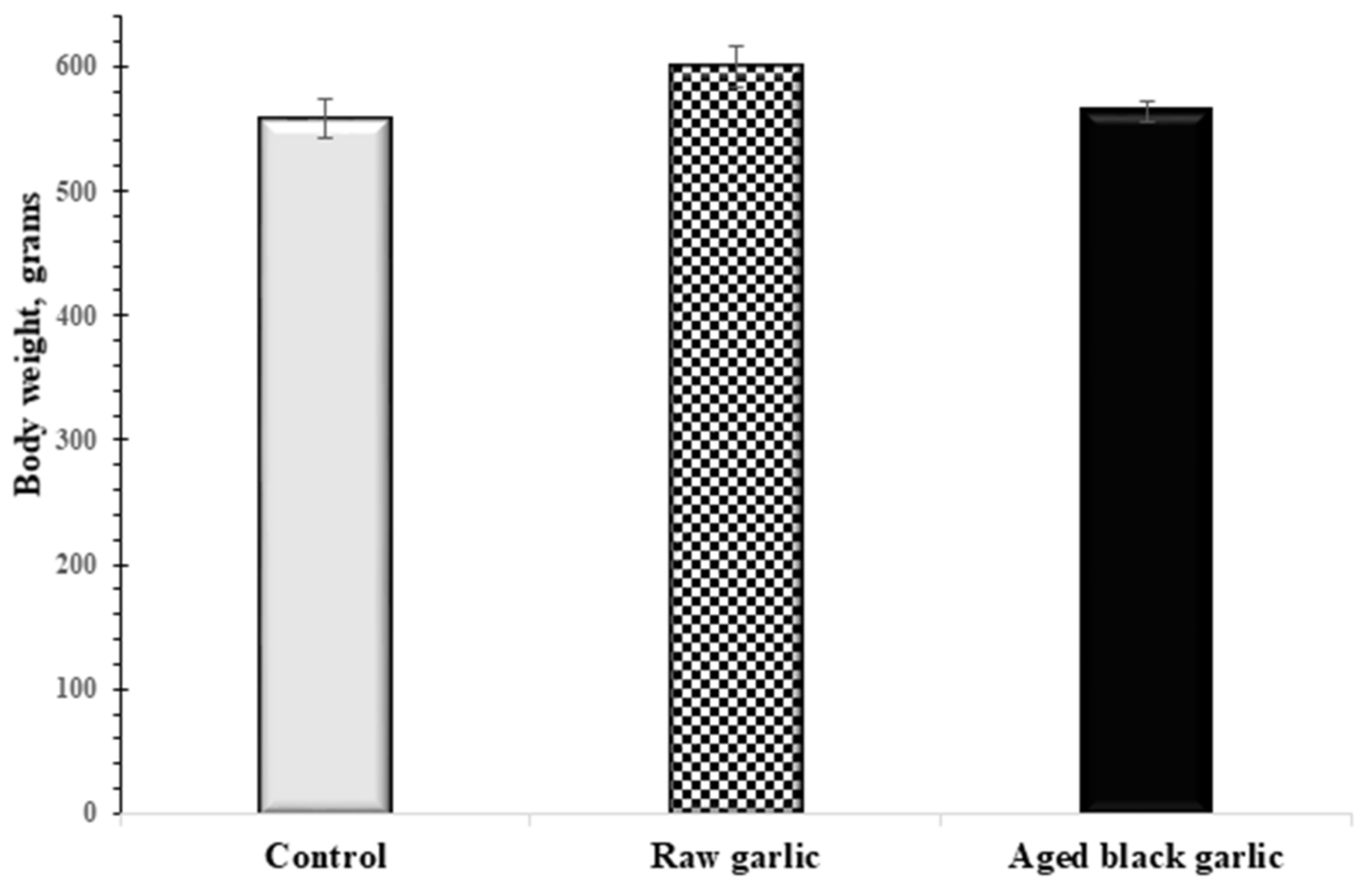
2.2. Effect of Raw and Aged Garlic on Body Weight and Blood Enzymes
2.3. Raw and Aged Garlic Treatment Protect the Heart from Ischemia/Reperfusion Injury
2.4. Infarct Size Reduction by Raw and Aged Black Garlic Treatment in Ischemic Reperfused Myocardium
2.5. Induction of HO-1 and iNOS by Garlic
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatment Protocol
4.3. Preparation of Aged Garlic
4.4. GC-MS Analyses
4.5. Isolated Working Heart Preparation and Cardiac Function Assessments
4.6. Infarct Size Measurements
4.7. Blood Enzymes
4.8. Western Blot Analyses
4.9. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, S.H.; Cho, Y.J.; Pan, J.H.; Cho, H.T.; Kim, J.H.; Bong, H.; Lee, Y.; Chang, M.H.; Jeong, Y.J.; et al. Preparation of S-Allylcysteine-Enriched Black Garlic Juice and Its Antidiabetic Effects in Streptozotocin-Induced Insulin-Deficient Mice. J. Agric. Food Chem. 2017, 65, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Kannel, W.B.; Castelli, W.P.; McNamara, P.M.; McKee, P.A.; Feinleib, M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N. Engl. J. Med. 1972, 287, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Aggarwal, A. Indigenous drugs in ischemic heart disease in patients with diabetes. J. Altern. Complement. Med. 2009, 15, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Papp, G.; Turoczi, T.; Varga, E.; Szendrei, L.; Vecsernyes, M.; Joo, F.; Tosaki, A. The role of heme oxygenase-related carbon monoxide and ventricular fibrillation in ischemic/reperfused hearts. Free Radic. Biol. Med. 2002, 33, 639–648. [Google Scholar] [CrossRef]
- Czompa, A.; Gyongyosi, A.; Czegledi, A.; Csepanyi, E.; Bak, I.; Haines, D.D.; Tosaki, A.; Lekli, I. Cardioprotection afforded by sour cherry seed kernel: The role of heme oxygenase-1. J. Cardiovasc. Pharmacol. 2014, 64, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, Y.; Okumi, M.; Isaka, Y.; Tsutahara, K.; Abe, T.; Yazawa, K.; Ichimaru, N.; Matsumura, K.; Hyon, S.H.; Takahara, S.; et al. Epigallocatechin-3-gallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Transpl. Int. 2011, 24, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ito, K.; Sumi, S.I.; Fuwa, T.; Horie, T. Antiapoptosis action of aged garlic extract (AGE) protects epithelial cells from methotrexate induced injury. Gut 2005, 54, 1819–1820. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef] [PubMed]
- Imai, J.; Ide, N.; Nagae, S.; Moriguchi, T.; Matsuura, H.; Itakura, Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Medica 1994, 60, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Wongpornchai, S.; Sriseadka, T.; Choonvisase, S. Identification and quantitation of the rice aroma compound, 2-acetyl-1-pyrroline, in bread flowers (Vallaris glabra Ktze). J. Agric. Food Chem. 2003, 51, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Handoko, D.D.; Pather, L.; Methven, L.; Elmore, J.S. Evaluation of 2-acetyl-1-pyrroline in foods, with an emphasis on rice flavour. Food Chem. 2017, 232, 531–544. [Google Scholar] [CrossRef] [PubMed]
- García-Villalón, A.L.; Amor, S.; Monge, L.; Fernández, N.; Prodanov, M.; Muñoz, M.; Inarejos-García, A.M.; Granado, M. In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects. J. Funct. Foods 2016, 27, 189–200. [Google Scholar] [CrossRef]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Kuo, W.W.; Tsai, S.J.; Lii, C.K. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric. Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Rietz, B.; Isensee, H.; Strobach, H.; Makdessi, S.; Jacob, R. Cardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusion. Mol. Cell. Biochem. 1993, 119, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Dinda, A.K.; Manchanda, S.C.; Maulik, S.K. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol. 2002, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.K.; Maulik, M.; Mancahanda, S.C.; Dinda, A.K.; Gupta, S.K.; Maulik, S.K. Dose-dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci. 2002, 70, 1509–1518. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of Anti-Oxidant and Anti-Inflammatory Effects between Fresh and Aged Black Garlic Extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Gweon, O.C.; Seo, Y.J.; Im, J.; Kang, M.J.; Kim, M.J.; Kim, J.I. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2009, 3, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, K.S.; Vasanthi, A.H.; Gurusamy, N. Steroidal Saponin Diosgenin from Dioscorea bulbifera Protects Cardiac Cells from Hypoxia-reoxygenation Injury Through Modulation of Pro-survival and Pro-death Molecules. Pharmacogn. Mag. 2016, 12 (Suppl. 1), S14–S20. [Google Scholar] [PubMed]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiang, Y.; Chen, Y.; Tang, Y.; Zhang, Y. Ginsenoside Rg1 Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury via Activation of Nrf2/HO-1 Signaling and Inhibition of JNK. Cell. Physiol. Biochem. 2017, 44, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Issan, Y.; Kornowski, R.; Aravot, D.; Shainberg, A.; Laniado-Schwartzman, M.; Sodhi, K.; Abraham, N.G.; Hochhauser, E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS ONE 2014, 9, e92246. [Google Scholar] [CrossRef] [PubMed]
- Lescano de Souza Junior, A.; Mancini Filho, J.; Pavan Torres, R.; Irigoyen, M.C.; Curi, R. Pretreatment with fish oil attenuates heart ischaemia consequences in rats. Exp. Physiol. 2017, 102, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Lee, P.C.; Zhang, Y.; Ho, C.; Griffith, B.P.; Shears, L.L., 2nd; Billiar, T.R. Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 2000, 101, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Doods, H.; Wu, D. Sabiporide reduces ischemia-induced arrhythmias and myocardial infarction and attenuates ERK phosphorylation and iNOS induction in rats. BioMed Res. Int. 2013, 2013, 504320. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, D.; Li, X.; Yang, N.; Guo, P.; Xu, D.; Li, X. Renoprotective effects of propofol on the expression of iNOS protein in rats with ischemia reperfusion injury. Int. J. Clin. Exp. Med. 2015, 8, 776–780. [Google Scholar] [PubMed]
- Granado, M.; Fernandez, N.; Monge, L.; Figueras, J.C.; Carreno-Tarragona, G.; Amor, S.; Garcia-Villalon, A.L. Effects of coronary ischemia-reperfusion in a rat model of early overnutrition. Role of angiotensin receptors. PLoS ONE 2013, 8, e54984. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, F.R.; Gres, P.; Boengler, K.; Duschin, A.; Konietzka, I.; Rassaf, T.; Snedovskaya, J.; Meyer, S.; Skyschally, A.; Kelm, M.; et al. Inducible nitric oxide synthase expression and cardiomyocyte dysfunction during sustained moderate ischemia in pigs. Circ. Res. 2008, 103, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, Y.K.; Takagi, G.; Huang, C.H.; Geng, Y.J.; Vatner, S.F. Enhanced iNOS function in myocytes one day after brief ischemic episode. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H423–H428. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bae, E.H.; Ma, S.K.; Kim, S.W. Altered Nitric Oxide System in Cardiovascular and Renal Diseases. Chonnam Med. J. 2016, 52, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Toda, H. Coronary hemodynamic regulation by nitric oxide in experimental animals: Recent advances. Eur. J. Pharmacol. 2011, 667, 41–49. [Google Scholar] [CrossRef] [PubMed]





| Compounds | Retention Time (min) | Raw Garlic | Aged Black Garlic | Structures |
|---|---|---|---|---|
| 2-acetyl-1-pyrroline | 7.6 | − | + |  |
| diallyl disulfide | 8.0 | + | + |  |
| diallyl trisulfide | 9.1 | + | + |  |
| dipropil trisulfide | 11.6 | + | + |  |
| allicin | 11.7 | + | − |  |
| Blood Parameters | Control n = 8 | Raw Garlic n = 10 | Aged Black Garlic n = 9 |
|---|---|---|---|
| ALT (U/L) | 45.0 ± 3.3 | 47.1 ± 1.9 | 44.1 ± 1.4 |
| ALP (U/L) | 91.3 ± 9.2 | 90.2 ± 1.3 | 84.8 ± 4.4 |
| ASTL (U/L) | 89.1 ± 9.1 | 89.6 ± 3.8 | 87.9 ± 4.3 |
| CHO (mmol/L) | 1.30 ± 0.12 | 1.41 ± 0.07 | 1.58 ± 0.05 * |
| LDL (mmol/L) | 0.224 ± 0.020 | 0.294 ± 0.019 * | 0.350 ± 0.021 * |
| TRIG (mmol/L) | 1.35 ± 0.23 | 1.07 ± 0.12 | 1.32 ± 0.10 |
| HDL (mmol/L) | 1.09 ± 0.11 | 1.19 ± 0.04 | 1.25 ± 0.06 |
| LDH (U/L) | 811 ± 173 | 772 ± 88 | 801 ± 82 |
| CRP (mg/L) | 1.35 ± 0.23 | 1.07 ± 0.12 | 1.32 ± 0.10 |
| CK–MB (U/L) | 765 ± 154 | 656 ± 66 | 746 ± 89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czompa, A.; Szoke, K.; Prokisch, J.; Gyongyosi, A.; Bak, I.; Balla, G.; Tosaki, A.; Lekli, I. Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications. Int. J. Mol. Sci. 2018, 19, 1017. https://doi.org/10.3390/ijms19041017
Czompa A, Szoke K, Prokisch J, Gyongyosi A, Bak I, Balla G, Tosaki A, Lekli I. Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications. International Journal of Molecular Sciences. 2018; 19(4):1017. https://doi.org/10.3390/ijms19041017
Chicago/Turabian StyleCzompa, Attila, Kitti Szoke, Jozsef Prokisch, Alexandra Gyongyosi, Istvan Bak, Gyorgy Balla, Arpad Tosaki, and Istvan Lekli. 2018. "Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications" International Journal of Molecular Sciences 19, no. 4: 1017. https://doi.org/10.3390/ijms19041017




