Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential Against Cyclophosphamide Induced Toxicity in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Drugs, Reagents and Instrumentations
2.2. Preparation, Extraction and Fractionation of EG Leaf Extract as well as Chromatographic Isolation
2.3. HPLC-DAD-ESI-MS/MS Conditions
2.4. Animals
2.5. Experimental Design
2.6. Determination of Liver and Kidney Toxicity Indices
2.7. Determination of Oxidative/Nitrosative Stress Markers and Protein Carbonyl in Liver and Kidney Tissues
2.8. Determination of Nrf2/HO-1 Pathway Activation in Liver and Kidney Tissues
| Nrf2 F: TTGTAGATGACCATGAGTCGC |
| R: TGTCCTGCTGTATGCTGCTT |
| Β-actin F: AGGAGTACGATGAGTCCGGC |
| R: CGCAGCTCAGTAACAGTCCG |
2.9. Determination of Pro-inflammatory Markers and Caspase-3 in Liver and Kidney Tissues
2.10. Histopathological Examination
2.11. Statistical Analysis
3. Results
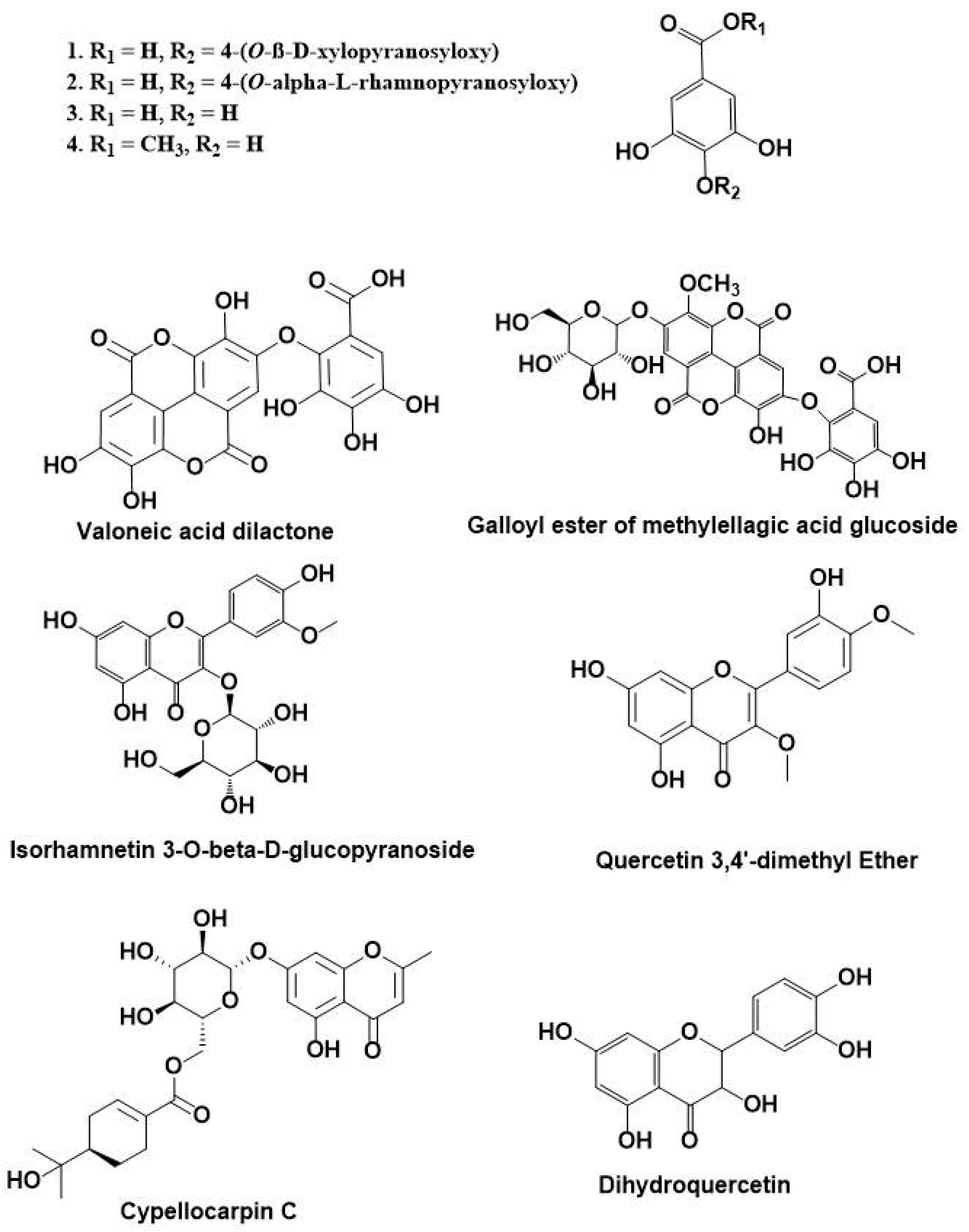
3.1. HPLC-DAD-ESI-MS-MS Annotation and Chromatographic Isolation of Polyphenolic Compounds
3.2. EG Pretreatment Alleviated CP Induced Liver and Kidney Damage
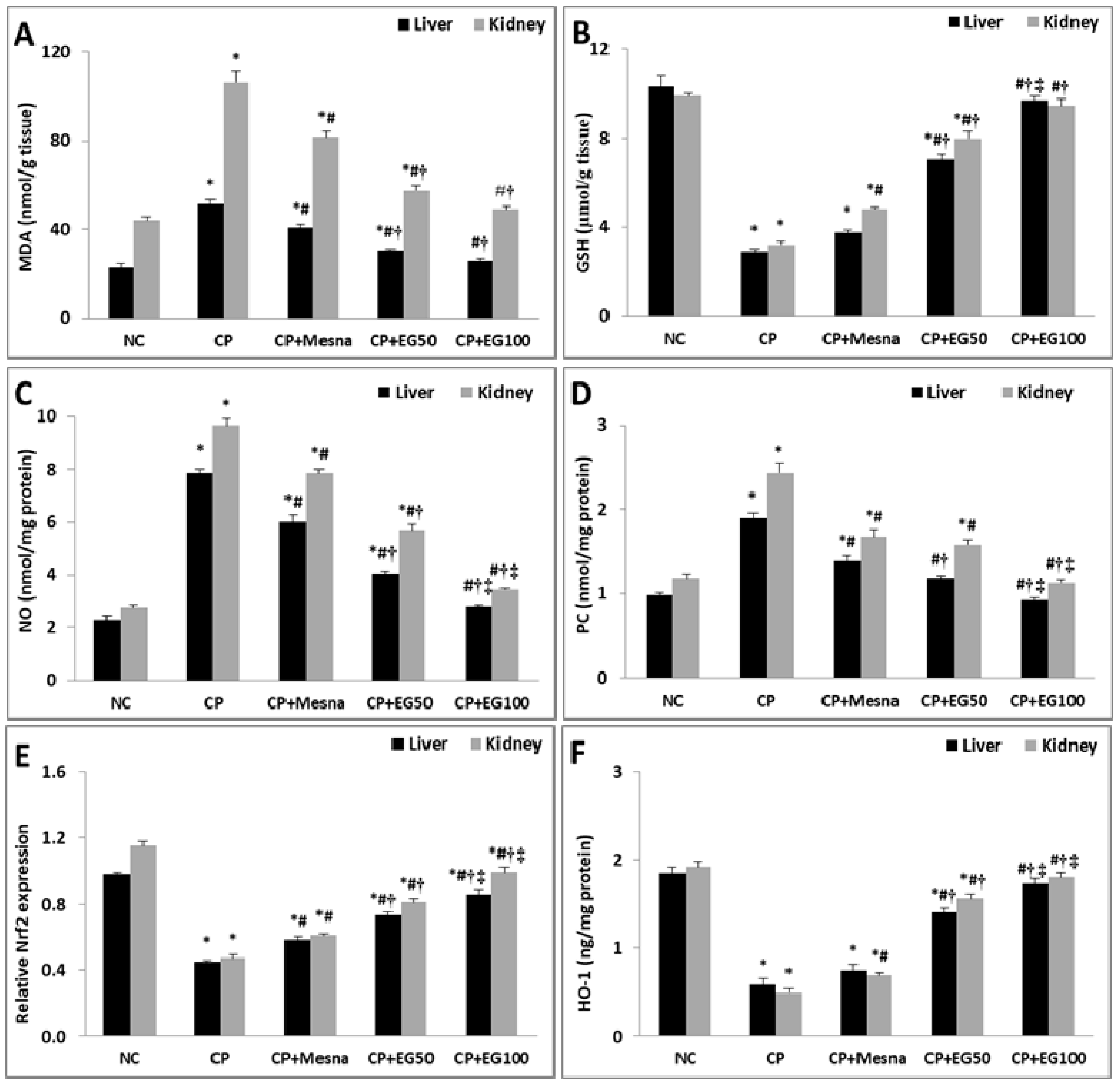
3.3. EG Pretreatment Mitigated CP-induced Oxidative/Nitosative Stress and Protein Carbonylation in Liver and Kidney Tissues
3.4. EG Pretreatment Activated Nrf2/HO-1/Antioxidant Signaling in The Livers and Kidneys of CP Treated Mice
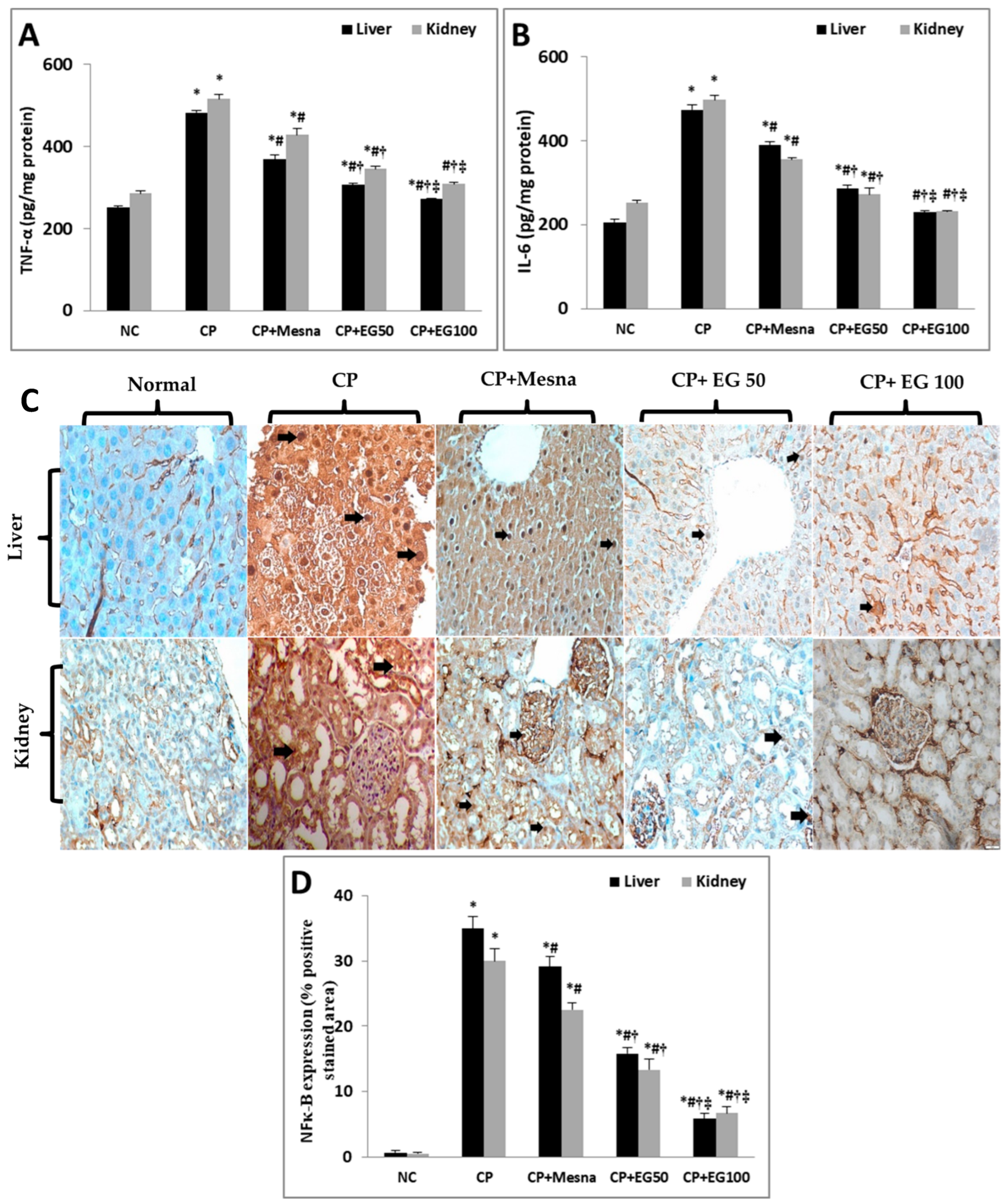
3.5. EG Pretreatment Down-regulated CP-induced Inflammation in Liver and Kidney Tissues
3.6. EG Blocked CP-induced Apoptosis in the Liver and Kidney of Mice
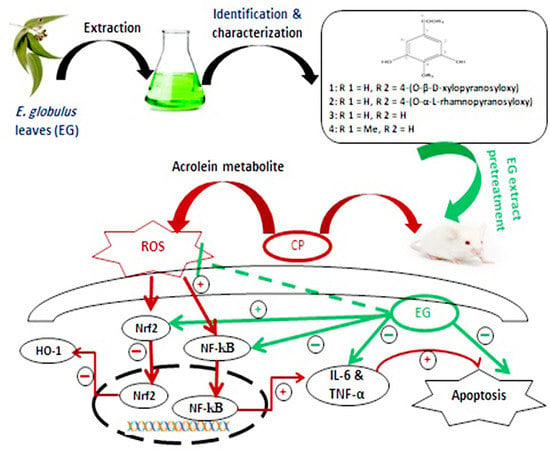
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC-DAD-ESI-MS/MS | High performance liquid chromatography coupled with diode array detection (DAD) and electrospray mass spectrometry (MS) |
| EG | Eucalyptus globulus |
| CP | Cyclophosphamide |
| NF-κB | Nuclear factor kappa-B |
| PC | Protein carbonylation |
| Nrf2 | Nuclear factor E2-related factor 2 |
| HO-1 | Hemoxygenase-1 |
| (IL)-6 | Interleukin |
| (TNF)-α | Tumor necrosis factor |
| ROS | Reactive oxygen species |
| Mesna | (2-mercaptoethane sulphonic acid) |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate buffered saline |
| DME | Defatted methanol extract |
| PC | Paper chromatography |
| UV | Ultra-violet |
| CC | Column chromatography |
| BIW | Butanol: isopropanol: water |
| i.p. | Intraperitoneally |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| BUN | Blood urea nitrogen |
| qRT-PCR | Quantitative reverse transcriptase real time polymerase chain reaction |
| H&E | Hematoxylin/eosin |
| MDA | Malondialdehyde |
| iNOS | Inducible nitric oxide synthase |
| US FDA | United States Food and Drug Administration |
| NO | Nitric oxide |
References
- Rehman, M.U.; Tahir, M.; Ali, F.; Qamar, W.; Lateef, A.; Khan, R.; Quaiyoom, A.; Oday-O-Hamiza; Sultana, S. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: The protective effect of ellagic acid. Mol. Cell. Biochem. 2012, 365, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Cuce, G.; Çetinkaya, S.; Koc, T.; Esen, H.H.; Limandal, C.; Balci, T.; Kalkan, S.; Akoz, M. Chemoprotective effect of vitamin E in cyclophosphamide induced hepatotoxicity in rats. Chem. Biol. Interact. 2015, 232, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Bhattacharjee, A.; Samanta, A.; Bhattacharya, S. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: Effect of an l-cysteine based oxovanadium (IV) complex on oxidative stress and DNA damage. Environ. Toxicol. Pharm. 2015, 40, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Bhattacharya, S. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol. Cell. Biochem. 2015, 405, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Hamsa, T.P.; Kuttan, G. Protective role of Ipomoea obscura (L.) on cyclophosphamide-induced uro- and nephrotoxicities by modulating antioxidant status and pro-inflammatory cytokine levels. Inflammopharmacology 2011, 19, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, J.; Li, P.; Lu, Q.; Pei, X.; Sun, Y.; Wang, G.; Hao, K. Magnesium isoglycyrrhizinate shows hepatoprotective effects in a cyclophosphamide-induced model of hepatic injury. Oncotarget 2017, 8, 33252–33264. [Google Scholar] [CrossRef] [Green Version]
- Zarei, M.; Shivanandappa, T. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chem. Toxicol. 2013, 57, 179–184. [Google Scholar] [CrossRef]
- Olayinka, E.; Ore, A.; Ola, O.; Adeyemo, O.A. Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats. Med. Sci. 2015, 3, 78–92. [Google Scholar] [CrossRef]
- Gunes, S.; Ayhanci, A.; Sahinturk, V.; Altay, D.U.; Uyar, R. Carvacrol attenuates cyclophosphamide-induced oxidative stress in rat kidney. Can. J. Physiol. Pharm. 2017, 95, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Shokrzadeh, M.; Ahmadi, A.; Naghshvar, F.; Chabra, A.; Jafarinejhad, M. Prophylactic efficacy of melatonin on cyclophosphamide-induced liver toxicity in mice. BioMed Res. Int. 2014, 2014, 470425. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, H.; Takao, T.; Yamamoto, K.; Nakayama, J.; Miyagawa, Y.; Tsujimura, A.; Nonomura, N.; Okuyama, A. Sesquiterpene lactone parthenolide ameliorates bladder inflammation and bladder overactivity in cyclophosphamide induced rat cystitis model by inhibiting nuclear factor-kappaB phosphorylation. J. Urol. 2009, 181, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hao, G.; Long, M.; Lai, F.; Li, Q.; Xiong, Y.; Tian, Y.; Lai, D. Oyster (Ostrea plicatula Gmelin) polysaccharides intervention ameliorates cyclophosphamide-Induced genotoxicity and hepatotoxicity in mice via the Nrf2—ARE pathway. Biomed. Pharm. 2017, 95, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- ALHaithloul, H.A.S.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharm. 2019, 111, 676–685. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (2009). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/20-855_Mesnex_Prntlbl.pdf (accessed on 29 July 2017).
- Yilmaz, N.; Emmungil, H.; Gucenmez, S.; Ozen, G.; Yildiz, F.; Balkarli, A.; Kimyon, G.; Coskun, B.N.; Dogan, I.; Pamuk, O.N.; et al. Incidence of cyclophosphamide-induced urotoxicity and protective effect of Mesna in rheumatic diseases. J. Rheumatol. 2015, 42, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Envrion. Sci. Pollut. Res. Int. 2018, 25, 20968–20984. [Google Scholar] [CrossRef] [PubMed]
- Cerig, S.; Geyikoglu, F.; Bakir, M.; Colak, S.; Sonmez, M.; Koc, K. Hepatoprotective effect of oleuropein against cisplatin-induced liver damage in rat. World Acad. Sci. Eng. Technol. 2016, 10, 260–267. [Google Scholar] [CrossRef]
- Sherif, I.O. The effect of natural antioxidants in cyclophosphamide-induced hepatotoxicity: Role of Nrf2/HO-1 pathway. Int. Immunopharmacol. 2018, 61, 29–36. [Google Scholar] [CrossRef]
- Murali, V.P.; Kuttan, G. Enhancement of cancer chemotherapeutic efficacy of cyclophosphamide by Curculigo orchioides Gaertn and its ameliorative effects on cyclophosphamide-induced oxidative stress. Integr. Cancer Ther. 2015, 14, 172–183. [Google Scholar] [CrossRef]
- White, D.A.; McGrath, J.F.; Ryan, M.G.; Battaglia, M.; Mendham, D.S.; Kinal, J.; Downes, G.M.; Crombie, D.S.; Hunt, M.E. Managing for water-use efficient wood production in Eucalyptus globulus plantations. For. Ecol. Manag. 2014, 331, 272–280. [Google Scholar] [CrossRef]
- Nakhaee, A.; Bokaeian, M.; Saravani, M.; Farhangi, A.; Akbarzadeh, A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J. Clin. Biochem. 2009, 24, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M.; Friedrich, U.; Jenett-Siems, K. Cytotoxicity of plants used in traditional medicine in Yemen. Fitoterapia 2005, 76, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Chalmers, A.C.; Bhuyan, D.J.; Bowyer, M.C.; Scarlett, C.J. Botanical, phytochemical, and anticancer properties of the Eucalyptus species. Chem. Biodivers. 2015, 12, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Khatun, H.; Ghosh, S.; Ali, M.M.; Khanam, J.A. Bioassay of Eucalyptus extracts for anticancer activity against Ehrlich ascites carcinoma (eac) cells in Swiss albino mice. Asian Pac. J. Trop. Biomed. 2012, 2, 394–398. [Google Scholar] [CrossRef]
- Islam, F.; Khatun, H.; Khatun, M.; Ali, S.M.; Khanam, J.A. Growth inhibition and apoptosis of Ehrlich ascites carcinoma cells by the methanol extract of Eucalyptus camaldulensis. Pharm. Biol. 2014, 52, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Boulekbache-Makhlouf, L.; Meudec, E.; Mazauric, J.P.; Madani, K.; Cheynier, V. Qualitative and semi-quantitative analysis of phenolics in Eucalyptus globulus leaves by high-performance liquid chromatography coupled with diode array detection and electrospray ionisation mass spectrometry. Phytochem. Anal. 2013, 24, 162–170. [Google Scholar] [CrossRef]
- Dezsi, Ș.; Bădărău, A.S.; Bischin, C.; Vodnar, D.C.; Silaghi-Dumitrescu, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L. Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) K.D. Hill & L.A.S. Johnson leaves. Molecules 2015, 20, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.; Sobeh, M.; Wink, M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharm. Pharm. 2018, 70, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, P.; Kulurkar, P.; Singh, D.; Kumar, D.; Patial, V. Iridoid glycosides fraction from Picrorhiza kurroa attenuates cyclophosphamide-induced renal toxicity and peripheral neuropathy via PPAR-γ mediated inhibition of inflammation and apoptosis. Phytomedicine 2017, 36, 108–117. [Google Scholar] [CrossRef]
- Abdi, S.A.; Najmi, A.K.; Raisuddin, S. Cyclophosphamide-induced down-regulation of uroplakin II in the mouse urinary bladder epithelium is prevented by S-allyl cysteine. Basic Clin. Pharm. Toxicol. 2016, 119, 598–603. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Wyrepkowski, C.C.; da Costa, D.M.G.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; dos Santos, L.C. Characterization and quantification of the compounds of the ethanolic extract from Caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Mingo-Chornet, H.; Pérez-Alonso, J.J.; Paola-Naranjo, R.D.; González-Paramás, A.M.; Santos-Buelga, C. Preparation of quercetin glucuronides and characterization by HPLC–DAD-ESI/MS. Eur. Food Res. Technol. 2008, 227, 1069–1076. [Google Scholar] [CrossRef]
- Bhat, G.; Shawl, A.S.; Shah, Z.; Tantry, M. HPLC-DAD-ESI-MS/MS identification and characterization of major constituents of Iris crocea, Iris germanica and Iris spuria growing in Kashmir Himalayas, India. J. Anal. Bioanal. Tech. 2014, 5, 223. [Google Scholar] [CrossRef]
- Ghareeb, M.; Saad, A.; Ahmed, W.; Refahy, L.; Nasr, S. HPLCDAD-ESI-MS/MS characterization of bioactive secondary metabolites from Strelitzia nicolai leaf extracts and their antioxidant and anticancer activities In vitro. Phcog. Res. 2018, 10, 368–378. [Google Scholar] [CrossRef]
- Gordon, A.; Jungfer, E.; da Silva, B.A.; Maia, J.G.S.; Marx, F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the amazon region. J. Agric. Food Chem. 2011, 59, 7688–7699. [Google Scholar] [CrossRef]
- Matsunami, K.; Takamor, I.; Shinzato, T. Radical-scavenging activities of new megastigmane glucosides from Macaranga tanarius (L.) MULL.-ARG. Chem. Pharm. Bull. 2006, 54, 1403–1407. [Google Scholar] [CrossRef]
- Boulekbache-Makhlouf, L.; Meudec, E.; Chibane, M.; Mazauric, J.P.; Cheynier, V.; Slimani, S.; Henry, M.; Madani, K. Analysis of phenolic compounds in fruit of Eucalyptus globulus cultivated in Algeria by high-performance liquid chromatography diode array detection mass spectrometry. J. Agric. Food Chem. 2010, 58, 12615–12624. [Google Scholar] [CrossRef]
- Singab, A.; Ayoub, N.; Al-Sayed, E.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K. Phenolic constituents of Eucalyptus camaldulensis Dehnh, with potential antioxidant and cytotoxic activities. Rec. Nat. Prod. 2011, 5, 271–280. [Google Scholar]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (muscadine grapes) as determined by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bergmeier, S. Compositions of glucose transport inhibitors as antitumor agents. International PCT Patent WO 2011119866 A1 20110929, 29 September 2011. [Google Scholar]
- Schuster, B.; Winter, M.; Herrmann, K. 4-O-β-d-glucosides of hydroxybenzoic and hydroxycinnamic acids-their synthesis and determination in berry fruit and vegetable. Z. Naturforsch. 1986, 41, 511–520. [Google Scholar] [CrossRef]
- Eldahshan, O.A. Isolation and structure elucidation of phenolic compounds of Carob leaves grown in Egypt. Curr. Res. J. Biol. Sci. 2011, 3, 52–55. [Google Scholar]
- Ekaprasada, M.T.; Nurdin, H.; Ibrahim, S.; Hamidi, D. Antioxidant activity of methyl gallate isolated from the leaves of Toonasureni. Indones. J. Chem. 2009, 9, 457–460. [Google Scholar] [CrossRef]
- Choi, J.G.; Mun, S.H.; Chahar, H.S.; Bharaj, P.; Kang, O.H.; Kim, S.G.; Shin, D.W.; Kwon, D.Y. Methyl Gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS ONE 2014, 9, e102697. [Google Scholar] [CrossRef]
- Mansour, D.F.; Salama, A.A.A.; Hegazy, R.R.; Omara, E.A.; Nada, S.A. Whey protein isolate protects against cyclophosphamide-induced acute liver and kidney damage in rats. J. Appl. Pharm. Sci. 2017, 7, 111–120. [Google Scholar] [CrossRef]
- Kamel, E.M.; Mahmoud, A.M.; Ahmed, S.A.; Lamsabhi, A.M. A phytochemical and computational study on flavonoids isolated from Trifolium resupinatum L. and their novel hepatoprotective activity. Food Funct. 2016, 7, 2094–2106. [Google Scholar] [CrossRef]
- Zhu, H.; Long, M.H.; Wu, J.; Wang, M.M.; Li, X.Y.; Shen, H.; Xu, J.D.; Zhou, L.; Fang, Z.J.; Luo, Y.; et al. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci. Rep. 2015, 5, 17536. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharm. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Yousefipour, Z.; Ranganna, K.; Newaz, M.A.; Milton, S.G. Mechanism of acrolein-induced vascular toxicity. J. Physiol. Pharmacol. 2005, 56, 337–353. [Google Scholar] [PubMed]
- Srivastava, A.; Shivanandappa, T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010, 118, 411–417. [Google Scholar] [CrossRef]
- Ahlem, S.; Khaled, H.; Wafa, M.; Sofiane, B.; Mohamed, D.; Jean-Claude, M.; Abdelfattah, E.F. Oral administration of Eucalyptus globulus extract reduces the alloxan-induced oxidative stress in rats. Chem. Biol. Interact. 2009, 181, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dhibi, S.; Mbarki, S.; Elfeki, A.; Hfaiedh, N. Eucalyptus globulus extract protects upon acetaminophen-induced kidney damages in male rat. Bosn. J. Basic Med. Sci. 2014, 14, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Sobeh, M.; Rezq, S.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. HPLC-ESI-MS/MS profiling of polyphenolics of a leaf extract from Alpinia zerumbet (Zingiberaceae) and its anti-inflammatory, anti-nociceptive, and antipyretic activities in vivo. Molecules 2018, 23, 3238. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.O.; Sabry, O.M.; Ghareeb, M.A.; El-Shazly, A.M.; Wink, M. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci. Rep. 2018, 8, 9343. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S.; Brodsky, S.V.; Noiri, E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002, 61, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Al Dera, H.S. 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of PPARγ and Nrf2 upregulation. Genes Nutr. 2015, 10, 41. [Google Scholar] [CrossRef]
- Andersson, M.C.; Tobin, G.; Giglio, D. Cholinergic nitric oxide release from the urinary bladder mucosa in cyclophosphamide-induced cystitis of the anaesthetized rat. Br. J. Pharm. 2008, 153, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Tokyay, R.; Kaya, E.; Gur, E.; Tuncel, P.; Ozbek, R.; Ozturk, E. Prostaglandin synthetase inhibition reduces peritonitis-induced early liver oxidant stress. Surg. Today 1999, 29, 42–46. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Laamech, J.; El-Hilaly, J.; Fetoui, H.; Chtourou, Y.; Gouitaa, H.; Tahraoui, A.; Lyoussi, B. Berberis vulgaris L. effects on oxidative stress and liver injury in lead-intoxicated mice. J. Complement. Integr. Med. 2017, 14, 20150079. [Google Scholar] [CrossRef] [PubMed]
- Quirós, Y.; Blanco-Gozalo, V.; Sanchez-Gallego, J.I.; López-Hernandez, F.J.; Ruiz, J.; Perez de Obanos, M.P.; López-Novoa, J.M. Cardiotrophin-1 therapy prevents gentamicin-induced nephrotoxicity in rats. Pharm. Res. 2016, 107, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharm. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18β-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren. Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, J.; Li, Y.; Song, H. Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell. Physiol. Biochem. 2017, 41, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Shrotriya, S.; Surh, Y.J. Kolaviron inhibits dimethyl nitrosamine-induced liver injury by suppressing COX-2 and iNOS expression via NF-κB and AP-1. Life Sci. 2009, 84, 149–155. [Google Scholar] [CrossRef]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Kucukler, S.; Caglayan, C.; Gur, C.; Batil, A.A.; Gülçin, İ. Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: Biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J. Food Biochem. 2017, 41, e12398. [Google Scholar] [CrossRef]
- El-Kholy, A.A.; Elkablawy, M.A.; El-Agamy, D.S. Lutein mitigates cyclophosphamide induced lung and liver injury via NF-κB/MAPK dependent mechanism. Biomed. Pharm. 2017, 92, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.E.; Sun, X.; Kim, M.K.; Li, W.Y.; Lee, S.W.; Koppula, S.; Yu, S.H.; Kim, H.B.; Kang, T.B.; Lee, K.H. Eucalyptus globulus inhibits inflammasome-activated pro-inflammatory responses and ameliorate monosodium urate-induced peritonitis in murine experimental model. Am. J. Chin. Med. 2018, 46, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Tsamandas, A.C.; Thomopoulos, K.; Zolota, V.; Kourelis, T.; Karatzas, T.; Ravazoula, P.; Tepetes, K.; Petsas, T.; Karavias, D.; Karatza, C.; et al. Potential role of bcl-2 and bax mRNA and protein expression in chronic hepatitis type B and C: A clinicopathologic study. Mod. Pathol. 2003, 16, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Brock, N.; Pohl, J. Prevention of urotoxic side effects by regional detoxification with increased selectivity of oxazaphosphorine cytostatics. IARC Sci. Publ. 1986, 78, 269–279. [Google Scholar]
- Hensley, M.L.; Schuchter, L.M.; Lindley, C.; Meropol, N.J.; Cohen, G.I.; Broder, G.; Gradishar, W.J.; Green, D.M.; Langdon, R.J., Jr.; Mitchell, R.B.; et al. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J. Clin. Oncol. 1999, 17, 3333–3355. [Google Scholar] [CrossRef]
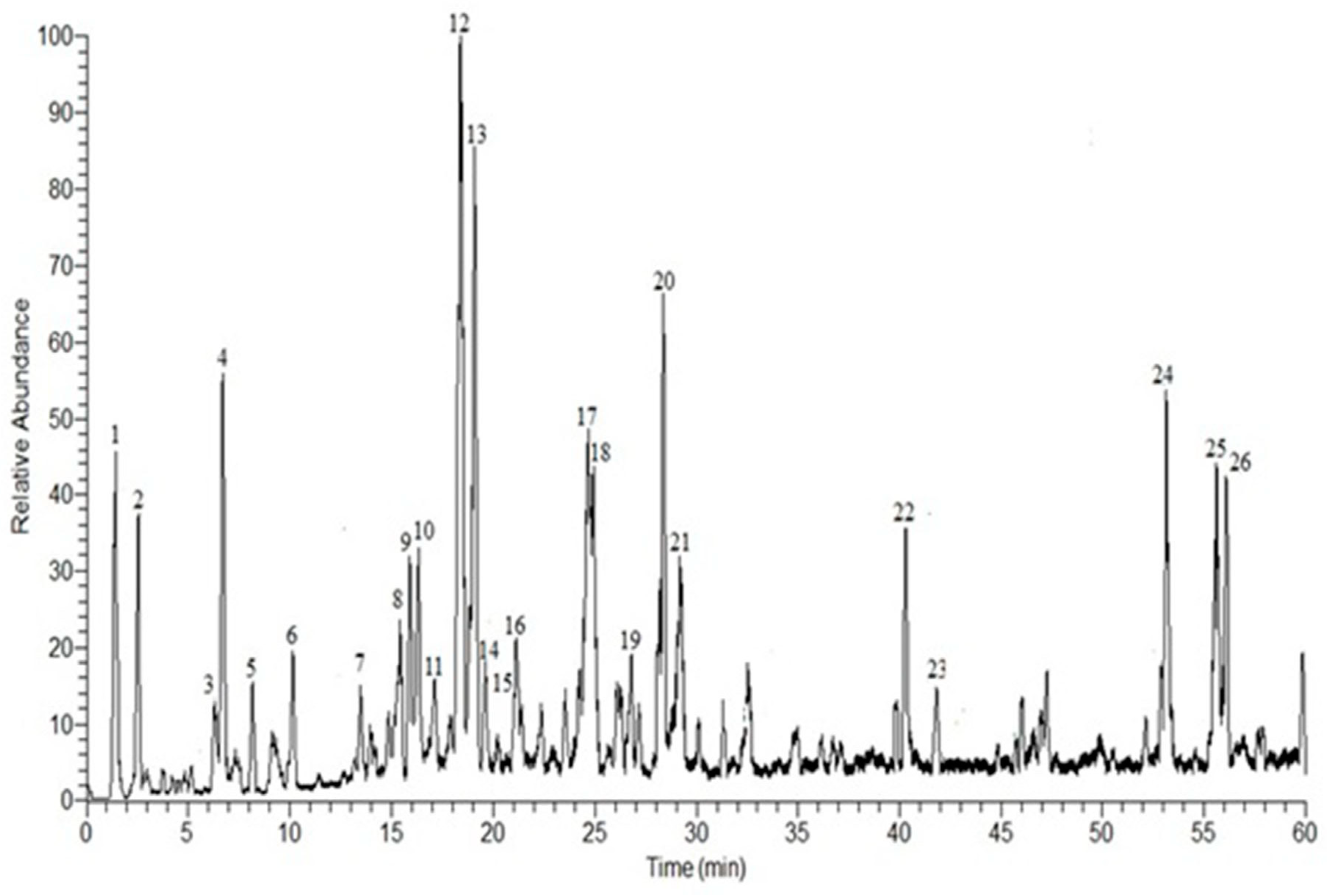


| No. | Rt | [M-H]− | Major Product Ions (m/z) | Tentative Identified Compounds * |
|---|---|---|---|---|
| 1 | 1.41 | 301 | 169, 125c | Gallic acid pentoside a,b |
| 2 | 2.54 | 315 | 169, 125 | Gallic acid rhamnoside a,b |
| 3 | 6.30 | 169 | 125 | Gallic acid a,b |
| 4 | 6.69 | 183 | 183, 169, 125 | Methyl gallate a,b |
| 5 | 8.16 | 353 | 191, 161 | Chlorogenic acid |
| 6 | 10.46 | 267 | 251, 223, 221, 205, 203, 193, 97, 85 | Unidentified |
| 7 | 13.55 | 537 | 313, 271, 211, 169 | Mallophenol B |
| 8 | 15.89 | 521 | 491, 359, 179 | Rosmarinic acid hexoside |
| 9 | 16.29 | 483 | 331, 271, 211, 169 | Digalloylglucose |
| 10 | 17.16 | 421 | 331, 313, 169, 151, 125 | Benzyl-galloylglucose |
| 11 | 17.50 | 481 | 463, 301, 271, 151 | Hexahydroxydiphenoyl-glucose |
| 12 | 18.68 | 491 | 473, 431, 315, 301, 179 | Isorhamnetin 3-O-β-d-glucuronoside |
| 13 | 19.64 | 689 | 537, 519, 211, 193 | Galloyl cypellocarpin B |
| 14 | 19.82 | 939 | 769, 635, 617, 599, 465 | Pentagalloylglucose |
| 15 | 20.12 | 635 | 483, 465, 423, 331, 169 | Trigalloylglucose |
| 16 | 21.54 | 625 | 473, 463, 437, 301, 257 | HHDP-diglucoside |
| 17 | 24.91 | 629 | 477, 315, 301 | Galloyl ester of a methylellagic acid glucoside |
| 18 | 25.31 | 329 | 329, 314, 301, 300, 299, 285, 243 | Quercetin-3,4’-dimethyl ether |
| 19 | 27.17 | 1085 | 765, 633, 473 | Eucalbanin A or cornusiin B |
| 20 | 28.52 | 519 | 353, 335, 233 | Cypellocarpin C |
| 21 | 29.61 | 1415 | 1113, 933, 783, 633 | Di (HHDP-galloylglucose)-pentose |
| 22 | 40.34 | 303 | 301, 285, 259, 179, 125 | Dihydroquercetin (Taxifolin) |
| 23 | 41.82 | 617 | 465, 343, 303, 169 | Trigalloyllevoglucosan |
| 24 | 53.13 | 953 | 635, 301, 169 | Valoneoyl-digalloyl-glucopyranose |
| 25 | 55.60 | 311 | 296, 293, 195 | Eicosanoic acid |
| 26 | 56.04 | 469 | 425, 423, 301, 169 | Valoneic acid dilactone |
| Animal Groups | Liver Functions | Kidney Functions | ||
|---|---|---|---|---|
| ALT | AST | Creatinine | BUN | |
| Normal Control | 23.65 ± 0.80 | 42.38 ± 1.27 | 0.26 ± 0.02 | 11.97 ± 0.47 |
| CP | 70.17 ± 1.65 * | 152.39 ± 1.34 * | 0.49 ± 0.02 * | 40.40 ± 1.71 * |
| CP + mesna | 60.11 ± 1.60 *# | 126. 26 ± 1.33 *# | 0.39 ± 0.01 *# | 31.71 ± 0.91 *# |
| CP + EG (50 mg/kg) | 33.68 ± 0.90 *#† | 61.11 ± 1.23 *#† | 0.34 ± 0.01 *† | 25.68 ± 0.65 *#† |
| CP + EG (100 mg/kg) | 24.78 ± 0.65 #†‡ | 46. 10 ± 0.96 #†‡ | 0.29 ± 0.01 #†‡ | 16.29 ± 0.52 *#†‡ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghareeb, M.A.; Sobeh, M.; El-Maadawy, W.H.; Mohammed, H.S.; Khalil, H.; Botros, S.; Wink, M. Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential Against Cyclophosphamide Induced Toxicity in Mice. Antioxidants 2019, 8, 415. https://doi.org/10.3390/antiox8090415
Ghareeb MA, Sobeh M, El-Maadawy WH, Mohammed HS, Khalil H, Botros S, Wink M. Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential Against Cyclophosphamide Induced Toxicity in Mice. Antioxidants. 2019; 8(9):415. https://doi.org/10.3390/antiox8090415
Chicago/Turabian StyleGhareeb, Mosad A., Mansour Sobeh, Walaa H. El-Maadawy, Hala Sh. Mohammed, Heba Khalil, Sanaa Botros, and Michael Wink. 2019. "Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential Against Cyclophosphamide Induced Toxicity in Mice" Antioxidants 8, no. 9: 415. https://doi.org/10.3390/antiox8090415