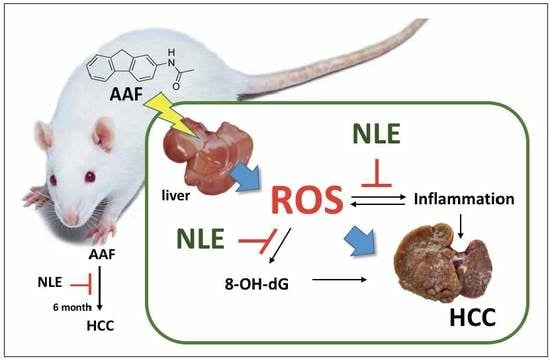
Inhibitory Effect of Nelumbo nucifera Leaf Extract on 2-Acetylaminofluorene-induced Hepatocarcinogenesis Through Enhancing Antioxidative Potential and Alleviating Inflammation in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of NLE and HPLC Analysis
2.3. Animals Maintenance and Treatment
2.4. Histopathological Examination for Malignant Hepatoma
2.5. Immunohistochemical Evaluation
2.6. Determination of Serum Biomarkers for Liver Fibrosis and Hepatocarcinogenesis
2.7. Measurement of Lipid Peroxidation and Antioxidant Enzymes
2.8. Western Blot Analysis
2.9. Assay of Serum IL-6 and TNF-α
2.10. Statistical Analysis
3. Results
3.1. Identification of NLE
3.2. Effect of NLE Supplementation on AAF-Induced Fibrosis
3.3. Body and Liver Weight and Exterior Examination
3.4. Blood Biochemical Study for Hepatic Injury
3.5. NLE Supplementation Inhibits AAF-Induced Inflammatory Mediators
3.6. Effect of NLE on AAF-Induced Hepatocarcinogenesis
3.7. NLE Supplementation Enhances Antioxidant Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altekruse, S.F.; McGlynn, K.A.; Kickie, L.A.; Kleiner, D.E. Hepatocellular carcinoma confirmation, treatment, and survival insurveillance, epidemiology, and end results registries, 1992–2008. Hepatology 2012, 55, 476–482. [Google Scholar] [CrossRef]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, J.E.; Chung, H.Y.; Choi, J.S. Antioxidant principles of Nelumbo nucifera stamens. Arch. Pharm. Res. 2003, 26, 279–285. [Google Scholar] [CrossRef]
- Ling, Z.Q.; Xie, B.J.; Yang, E.L. Isolation, characterization, and determination of antioxidative activity of oligomeric procyanidins from the seedpod of Nelumbo nucifera Gaertn. J. Agric. Food Chem. 2005, 53, 2441–2445. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Das, J.; Saha, K.; Giri, S.; Pal, M.; Saha, B. Antipyretic activity of Nelumbo nucifera rhizome extract. Indian J. Exp. Biol. 1996, 34, 275–276. [Google Scholar]
- Mukherjee, P.K.; Saha, K.; Pal, M.; Saha, B. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J. Ethnopharmacol. 1997, 58, 207–213. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef]
- Ho, H.H.; Hsu, L.S.; Chan, K.C.; Chen, H.M.; Wu, C.H.; Wang, C.J. Extract from the leaf of nucifera reduced the development of atherosclerosis via inhibition of vascular smooth muscle cell proliferation and migration. Food Chem. Toxicol. 2010, 48, 159–168. [Google Scholar] [CrossRef]
- Wu, C.H.; Yang, M.Y.; Chan, K.C.; Chung, P.J.; Ou, T.T.; Wang, C.J. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J. Agric. Food Chem. 2010, 58, 7075–7081. [Google Scholar] [CrossRef]
- Tang, C.C.; Lin, W.L.; Lee, Y.J.; Tang, Y.C.; Wang, C.J. Polyphenol-rich extract of Nelumbo nucifera leaves inhibits alcohol-induced steatohepatitis via reducing hepatic lipid accumulation and anti-inflammation in C57BL/6J mice. Food Funct. 2014, 5, 678–687. [Google Scholar] [CrossRef]
- Chang, C.H.; Ou, T.T.; Yang, M.Y.; Huang, C.C.; Wang, C.J. Nelumbo nucifera Gaertn leaves extract inhibits the angiogenesis and metastasis of breast cancer cells by downregulation connective tissue growth factor (CTGF) mediated PI3K/AKT/ERK signaling. J. Ethnopharmacol. 2016, 188, 111–122. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chang, Y.C.; Chan, K.C.; Lee, Y.J.; Wang, C.J. Flavonoid-enriched extracts from Nelumbo nucifera leaves inhibits proliferation of breast cancer in vitro and in vivo. Eur. J. Integr. Med. 2011, 3, e153–e163. [Google Scholar] [CrossRef]
- Lin, M.C.; Kao, S.H.; Chung, P.J.; Chan, K.C.; Yang, M.Y.; Wang, C.J. Improvement for high fat diet-induced hepatic injuries and oxidative stress by flavonoid-enriched extract from Nelumbo nucifera leaf. J. Agric. Food Chem. 2009, 57, 5925–5932. [Google Scholar] [CrossRef]
- Lima, K.G.; Krause, G.C.; Schuster, A.D.; Catarina, A.V.; Basso, B.S.; De Mesquita, F.C.; Pedrazza, L.; Marczak, E.S.; Martha, B.A.; Nunes, F.B. Gallic acid reduces cell growth by induction of apoptosis and reduction of IL-8 in HepG2 cells. Biomed. Pharmacother. 2016, 84, 1282–1290. [Google Scholar] [CrossRef]
- Jagan, S.; Ramakrishnan, G.; Anandakumar, P.; Kamaraj, S.; Devaki, T. Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol. Cell. Biochem. 2008, 319, 51. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Kochi, T.; Ideta, T.; Miyazaki, T.; Moriwaki, H. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int. J. Mol. Sci. 2015, 16, 6124–6139. [Google Scholar] [CrossRef]
- Choi, S.; Lim, T.G.; Hwang, M.K.; Kim, Y.A.; Kim, J.; Kang, N.J.; Jang, T.S.; Park, J.S.; Yeom, M.H.; Lee, K.W. Rutin inhibits B[a]PDE-induced cyclooxygensase-2 expression by targeting EGFR kinase activity. Biochem. Pharmacol. 2013, 86, 1468–1475. [Google Scholar] [CrossRef]
- Chandra, Y.P.; Viswanathswamy, A. Chemopreventive effect of Rutin against N-nitrosodiethylamine-induced and phenobarbital-promoted hepatocellular carcinoma in Wistar rats. Indian J. Pharm. Educ. Res. 2018, 52, 78–86. [Google Scholar] [CrossRef]
- Huang, G.; Tang, B.; Tang, K.; Dong, X.; Deng, J.; Liao, L.; Liao, Z.; Yang, H.; He, S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol. Rep. 2014, 31, 2377–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.H.; Yang, M.Y.; Wang, C.J. Quercetin-3-O-glucuronide inhibits doxorubicin resistance by reducing endoplasmic reticulum stress in hepatocellular carcinoma cells. J. Funct. Foods 2019, 54, 301–309. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Zhang, L.; Cheng, Z.; Deng, X.; Shu, G. Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo. J. Agric. Food Chem. 2017, 65, 5961–5972. [Google Scholar] [CrossRef] [PubMed]
- Strom, S.C.; Jirtle, R.L.; Michalopoulos, G. Genotoxic effects of 2-acetylaminofluorene on rat and human hepatocytes. Environ. Health Perspect. 1983, 49, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Klöhn, P.C.; Massalha, H.; Neumann, H.G. A metabolite of carcinogenic 2-acetylaminofluorene, 2-nitrosofluorene, induces redox cycling in mitochondria. Biochim. Biophys. Acta Bioenergy 1995, 1229, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.D.; Kao, S.H.; Chang-Che Tu, C.C.; Li, Y.J.; Wang, C.J. Solanum nigrum L. Extract Inhibits 2-Acetylaminofluorene-Induced Hepatocarcinogenesis through Overexpression of Glutathione S-Transferase and Antioxidant Enzymes. J. Agric. Food Chem. 2009, 57, 8628–8634. [Google Scholar] [CrossRef]
- Bergmeyer, H.; Herder, M.; Rej, R. International Federation of Clinical Chemistry (IFCC). J. Clin. Chem. Clin. Biochem. 1986, 24, 497–510. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Lee, H.J.; Chen, C.C.; Chou, F.P.; Wu, C.H.; Lai, F.S.; Yang, M.Y.; Wang, C.J. Water extracts from Nelumbo nucifera leaf reduced plasma lipids and atherosclerosis in cholesterol-fed rabbits. J. Food Biochem. 2010, 34, 779–795. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Li, T.; Zhao, X.P.; Wang, L.Y.; Gao, S.; Zhao, J.; Fan, Y.C.; Wang, K. Glutathione S-transferase P1 correlated with oxidative stress in hepatocellular carcinoma. Int. J. Med. Sci. 2013, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Budzinska, M.; Maczurek, A.; Cheng, R.; Di Bartolomeo, A.; Warner, F.; McCaughan, G.; McLennan, S.; Shackel, N. Novel aspects of the liver microenvironment in hepatocellular carcinoma pathogenesis and development. Int. J. Mol. Sci. 2014, 15, 9422–9458. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. The role of phenolic compounds in the fight against cancer—A review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C.; Wang, M.Y. Effect of flavonoids from Lotus (Nelumbo nuficera Gaertn) leaf on biochemical parameters related to oxidative stress induced by exhaustive swimming exercise of mice. Biomed. Res. 2014, 25, 1–6. [Google Scholar]
- Park, E.; Kim, G.D.; Go, M.S.; Kwon, D.; Jung, I.K.; Auh, J.H.; Kim, J.H. Anti-inflammatory effects of Nelumbo leaf extracts and identification of their metabolites. Nutr. Res. Pract. 2017, 11, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Aglan, A.H.; Ahmed, H.H.; EI-Toumy, S.A.; Mahmoud, N.S. Gallic acid against hepatocellular carcinoma: An integrated scheme of the potential mechanisms of action from in vivo study. Tumor Biol. 2017, 39, 1010428317699127. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, J.M.; Sagar, V.M.; Shah, T.; Shetty, S. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J. Gastroenterol. 2018, 24, 4436–4447. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.-Y.; Hung, T.-W.; Wang, C.-J.; Tseng, T.-H. Inhibitory Effect of Nelumbo nucifera Leaf Extract on 2-Acetylaminofluorene-induced Hepatocarcinogenesis Through Enhancing Antioxidative Potential and Alleviating Inflammation in Rats. Antioxidants 2019, 8, 329. https://doi.org/10.3390/antiox8090329
Yang M-Y, Hung T-W, Wang C-J, Tseng T-H. Inhibitory Effect of Nelumbo nucifera Leaf Extract on 2-Acetylaminofluorene-induced Hepatocarcinogenesis Through Enhancing Antioxidative Potential and Alleviating Inflammation in Rats. Antioxidants. 2019; 8(9):329. https://doi.org/10.3390/antiox8090329
Chicago/Turabian StyleYang, Mon-Yuan, Tung-Wei Hung, Chau-Jong Wang, and Tsui-Hwa Tseng. 2019. "Inhibitory Effect of Nelumbo nucifera Leaf Extract on 2-Acetylaminofluorene-induced Hepatocarcinogenesis Through Enhancing Antioxidative Potential and Alleviating Inflammation in Rats" Antioxidants 8, no. 9: 329. https://doi.org/10.3390/antiox8090329