Effect of Alkaline Salts on Pyrolyzed Solid Wastes in Used Edible Oils: An Attenuated Total Reflectance Analysis of Surface Compounds as a Function of the Temperature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Raw Biomass
3.2. Characterization of Biochar Obtained via Pyrolysis
3.3. ATR-FTIR Analysis: The Effect of Temperature
3.4. Influence of the Inorganic Salts on the Pyrolysis
4. Discussion
- The presence of bands around 3000 cm−1, due to aliphatic hydrocarbons in the biochars obtained from the water-washed samples (not detected in the unwashed sample), evidences the incomplete decomposition of the initial material. Given that in similar conditions of pyrolysis, the degree of decomposition is lower when inorganic salts are eliminated, we can think that this is a catalytic effect of these salts, as previously reported in the literature [35,36].
- There is a strong decrease in the band at 1030 cm−1 in the biochar obtained from the water-washed samples, evidencing that inorganic salts stabilize oxygenated compounds on the biochar surface. The retention of oxygenated compounds on the solid surface will influence the O/C ratio in the other products (bio-oil and gas), which is a very interesting factor from the point of view of the utilization of these fractions in the energy field [37].
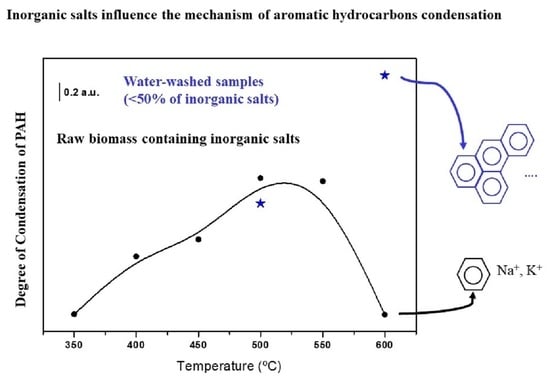
- The DOC calculated for the biochar obtained from the water-washed samples at T > 500 °C (Figure 6, blue trace) is much higher in the absence of inorganic salts. This fact clearly evidences the participation of NaCl (KCl) in the mechanism of PAH condensation, probably stabilizing the free radicals, intermediate of the condensation process, as indicated in schema 1. This fact was previously discussed in the literature [38].
5. Conclusions
- Inorganic salts have a catalytic effect on the sample thermal degradation at temperatures lower than 500 °C, but the DOC of the PAH is not strongly affected at these temperatures.
- In their presence, oxygenated groups are stabilized on the biochar surface. This aspect may affect the composition of gas and liquid fractions obtained via pyrolysis.
- These salts participate in the mechanism of PAH condensation at temperatures higher than 500 °C, causing a decrease in the DOC of PAHs in their presence.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gromada, A.; Trebska, P.; Wysokinski, P. Use of energy in Polish agriculture. In Proceedings of the 19th International Scientific Conference on Economic Science for Rural Development, Jelgava, Latvia, 9–11 May 2018. [Google Scholar] [CrossRef]
- Herc, L.; Pfeifer, A.; Duic, N. Optimization of the possible pathways for gradual energy system decarbonization. Renew. Energy 2022, 193, 617–633. [Google Scholar] [CrossRef]
- Din, M.I.; Ameen, S.; Hussain, Z.; Khalid, R.; Hussain, T.; Mujahid, A. A review on biomass selection criteria and its preliminary test for pyrolysis technique. Int. J. Environ. Anal. Chem. 2021, 101, 1–12. [Google Scholar] [CrossRef]
- Shukla, P.; Giri, B.S.; Mishra, R.K.; Pandey, A.; Chaturvedi, P. Lignocellulosic biomass-based engineered biochar composites: A facile strategy for abatement of emerging pollutants and utilization in industrial applications. Renew. Sustain. Energy Rev. 2021, 152, 111643. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Laird, D.A. The Charcoal Vision: A Win–Win–Win Scenario for Simultaneously Producing Bioenergy, Permanently Sequestering Carbon, while Improving Soil and Water Quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Wang, S.; Wang, N.; Yao, K.; Fan, Y.; Li, W.; Han, W.; Yin, X.; Chen, D. Characterization and Interpretation of Cd (II) Adsorption by Different Modified Rice Straws under Contrasting Conditions. Sci. Rep. 2019, 9, 17868. [Google Scholar] [CrossRef]
- Shan, R.; Shi, Y.; Gu, J.; Wang, Y.; Yuan, H. Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin. J. Chem. Eng. 2020, 28, 1375–1383. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Liu, Y.R.; Paskevicius, M.; Wang, H.Q.; Parkinson, G.; Wei, J.T.; Akhtar, M.A.; Li, C.Z. Insights into the mechanism of tar reforming using biochar as a catalyst. Fuel 2021, 296, 120672. [Google Scholar]
- Koutcheiko, S.; Monreal, C.M.; Kodama, H.; McCracken, T.; Kotlyar, L. Preparation and characterization of activated carbon derived from the thermo-chemical conversion of chicken manure. Bioresour. Technol. 2007, 98, 2459–2464. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Tournaki, S.; Gkouskos, Z.; Paraíba, O.; Giglio, F.; Quero García, P.; Braga, J.; Adrianos, H.; Filice, M. Quality Characteristics of Biodiesel Produced from Used Cooking Oil in Southern Europe. ChemEngineering 2019, 3, 19. [Google Scholar]
- Singh, E.; Mishra, R.; Kumar, A.; Shukla, S.K.; Lo, S.-L.; Kumar, S. Circular economy-based environmental management using biochar: Driving towards sustainability. Process. Saf. Environ. Prot. 2022, 163, 585–600. [Google Scholar]
- Muzyka, R.; Misztal, E.; Hrabak, J.; Banks, S.W.; Sajdak, M. Various biomass pyrolysis conditions influence the porosity and pore size distribution of biochar. Energy 2023, 263, 126128. [Google Scholar]
- Greco, G.; Videgain, M.; Di Stasi, C.; Pires, E.; Manya, J.J. Importance of pyrolysis temperature and pressure in the concentration of polycyclic aromatic hydrocarbons in wood waste-derived biochars. J. Anal. Appl. Pyrolysis 2021, 159, 105337. [Google Scholar]
- Palade, L.M.; Negoit, M.; Adascălului, A.C.; Mihai, A.L. Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review. Appl. Sci. 2023, 13, 7877. [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar]
- Pasieczna-Patkwoska, S.; Madej, J. Comparison of photoacoustic, diffuse reflectance, attenuated total reflectance and transmission spectroscopy for the study of biochars. Pol. J. Chem. Technol. 2018, 20, 75–83. [Google Scholar]
- Montiano, M.G.; Fernández, A.M.; Díaz-Faes, E.; Barriocanal, C. Tar from biomass/coal-containing briquettes. Evaluation of PAHs. Fuel 2015, 154, 261–267. [Google Scholar]
- Billaud, F.; Gornay, J.; Coniglio, L. Pyrolysis of secondary raw material from used frying oils. Récents Progrès Génie Procédés 2007, 94, 1–8. [Google Scholar]
- Yang, H.; Huan, B.; Chen, Y.; Gao, Y.; Li, J.; Chen, H. Biomass-based pyrolytic polygeneration system for bamboo industry waste: Evolution of the char structure and the pyrolysis mechanism. Energy Fuels 2016, 30, 6430–6439. [Google Scholar]
- Zhao, Y.; Feng, D.; Zhang, Y.; Huang, Y.; Sun, S. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process. Technol. 2016, 141, 54–60. [Google Scholar]
- Mishra, R.K.; Mohanty, K. Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifoliaseeds over metal oxide catalysts. Biomass Convers. Biorefin. 2020, 10, 621–637. [Google Scholar]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food. Chem. 2011, 59, 2501–2510. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar]
- Zhang, C.; Zhang, L.; Gao, J.; Zhang, S.; Liu, Q.; Duan, P. Evolution of the functional groups/structures of biochar and heteroatoms during the pyrolysis of seaweed. Algal Res. 2020, 48, 101900. [Google Scholar]
- Hernández, S.A.; Zacconi, F.C.M. Sweet almond oil: Extraction, characterization and application. Quim. Nova 2009, 35, 1342–1345. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Sato, H.; Ohya, H. A Method for Determining the Composition of Aqueous Highly Concentrated Salt Mixture Solutions by Attenuated Total-Reflectance IR Spectrometry. Anal. Sci. 1998, 14, 803–808. [Google Scholar]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb Immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar]
- Wang, M.; Chen, J.; Jing, B.; Zhang, L.; Dong, Y.; Yu, X. Analysis of Reaction Kinetics of Edible Oil Oxidation at Ambient Temperature by FTIR Spectroscopy. Eur. J. Lipid Sci. Technol. 2020, 122, 1900302. [Google Scholar] [CrossRef]
- Nor Azman, N.A.N.M.; Asmadi, M.; Amin, N.A.S.; Shamjuddin, A.; Zainol, M.M.; Phaiboonsilpa, N.; Kawamoto, H. Polycyclic Aromatic Hydrocarbons Occurrences in BiomassChar and Its Mitigation Approaches: A Mini Review. ChemBioEng Rev. 2023, 10, 462–479. [Google Scholar] [CrossRef]
- Obradovic, N.; Stevanovic, S.; Zeljkovic, V.; Ristic, M.M. Influence of ZnO specific surface area on its sintering kinetics. Powder Metall. Met. Ceram. 2009, 48, 182–185. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Analysis of the Catalytic Effects Induced by Alkali and Alkaline Earth Metals (AAEMs) on the Pyrolysis of Beech Wood and Corncob. Catalysts 2022, 12, 1505. [Google Scholar]
- He, J.J.; Li, J.Y.; Wang, R.P.; Fang, X.D.; Huang, Q.X.; Wang, S.K.; Long, J.S. Effect of Potassium Chloride on the Nanostructure and Reactivity of Soot Particles from Combustion of Solid Waste. Energy Fuels 2023, 37, 1247–1256. [Google Scholar] [CrossRef]
- Marinescu, M.; Popovici, D.R.; Bombos, D.; Vasilievici, G.; Rosca, P.; Oprescu, E.E.; Bolocan, I. Hydrodeoxygenation and hydrocracking of oxygenated compounds over CuPd/γ-Al2O3–ZSM-5 catalyst. React. Kinet. Mech. Catal. 2021, 133, 1013–1026. [Google Scholar]
- Feng, D.; Shang, Q.; Dong, H.; Zhang, Y.; Wang, Z.; Li, D.; Xie, M.; Wei, Q.; Zhao, Y.; Sun, S. Catalytic mechanism of Na on coal pyrolysis-derived carbon black formation: Experiment and DFT simulation. Fuel Process. Technol. 2021, 224, 107011. [Google Scholar]









| Sample | Temperature (°C) | C | H | O 1 | N | S | H/C 2 | O/C 2 |
|---|---|---|---|---|---|---|---|---|
| S-Initial | - | 50.66 | 7.15 | 39.73 | 2.36 | 0.11 | 1.69 | 0.56 |
| S-450 | 450 | 43.24 | 1.95 | 52.23 | 2.43 | 0.08 | 0.54 | 0.92 |
| S-500 | 500 | 41.31 | 1.28 | 54.64 | 2.68 | 0.09 | 0.37 | 0.99 |
| S-600 | 600 | 42.31 | 0.98 | 53.65 | 2.94 | 0.12 | 0.28 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Sarria, F.; Real, C.; Córdoba, J.M.; Hidalgo, M.C.; Alcalá, M.D. Effect of Alkaline Salts on Pyrolyzed Solid Wastes in Used Edible Oils: An Attenuated Total Reflectance Analysis of Surface Compounds as a Function of the Temperature. Spectrosc. J. 2023, 1, 98-110. https://doi.org/10.3390/spectroscj1020009
Romero-Sarria F, Real C, Córdoba JM, Hidalgo MC, Alcalá MD. Effect of Alkaline Salts on Pyrolyzed Solid Wastes in Used Edible Oils: An Attenuated Total Reflectance Analysis of Surface Compounds as a Function of the Temperature. Spectroscopy Journal. 2023; 1(2):98-110. https://doi.org/10.3390/spectroscj1020009
Chicago/Turabian StyleRomero-Sarria, Francisca, Concepción Real, José Manuel Córdoba, María Carmen Hidalgo, and María Dolores Alcalá. 2023. "Effect of Alkaline Salts on Pyrolyzed Solid Wastes in Used Edible Oils: An Attenuated Total Reflectance Analysis of Surface Compounds as a Function of the Temperature" Spectroscopy Journal 1, no. 2: 98-110. https://doi.org/10.3390/spectroscj1020009