The Influence of Dietary Synbiotic on Agonistic Behavior, Stress, and Brain Monoamines via Modulation of the Microbiota–Gut–Brain Axis in Laying Hens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Environment
2.3. Treatments
2.4. Behavior
2.5. Blood
2.6. Brain and Cecal Collection
2.6.1. Brain Sample Preparation
2.6.2. Determination of Brain Monoamines
2.6.3. Cecal Contents
3. Statistical Analysis
4. Results
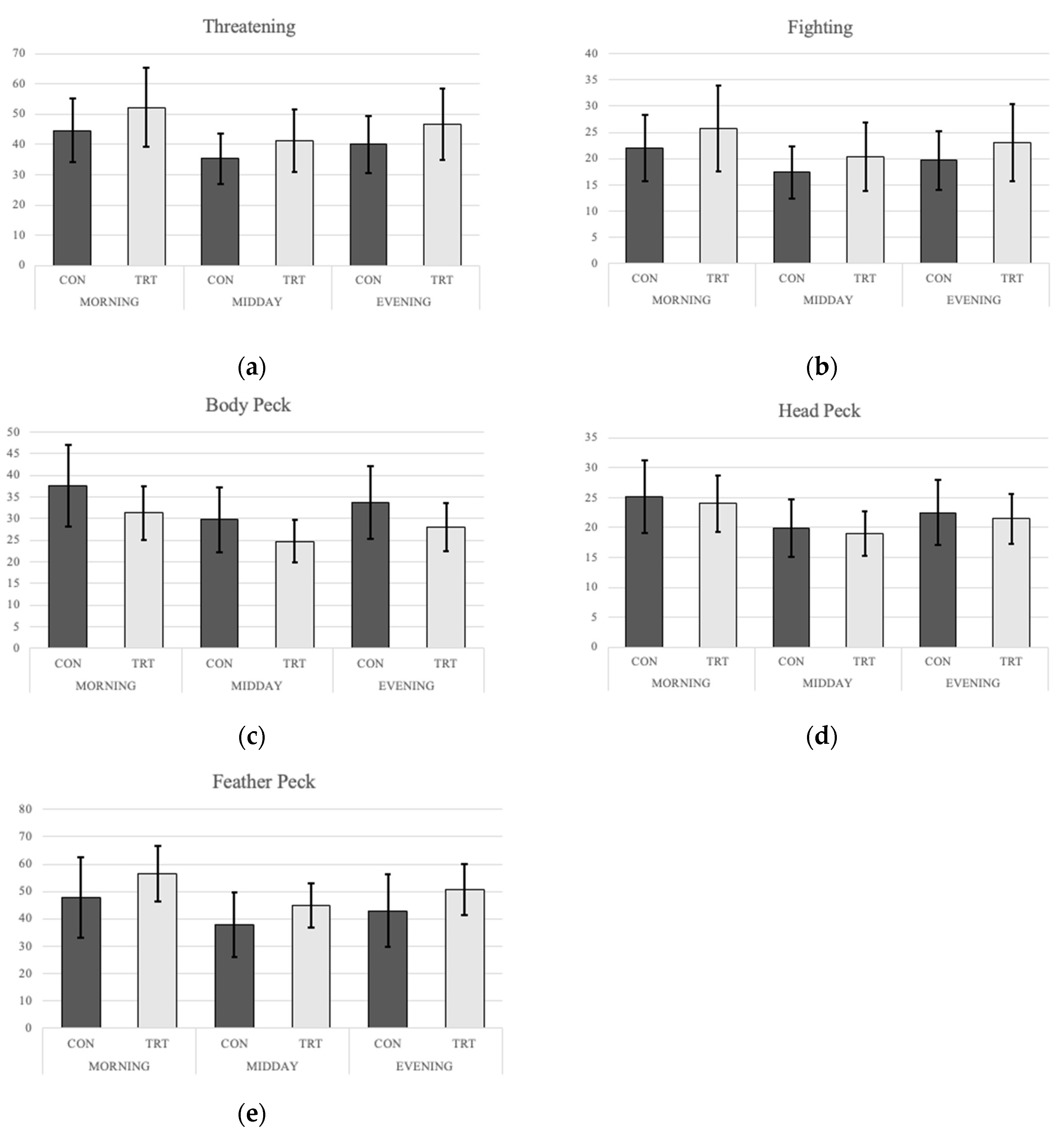
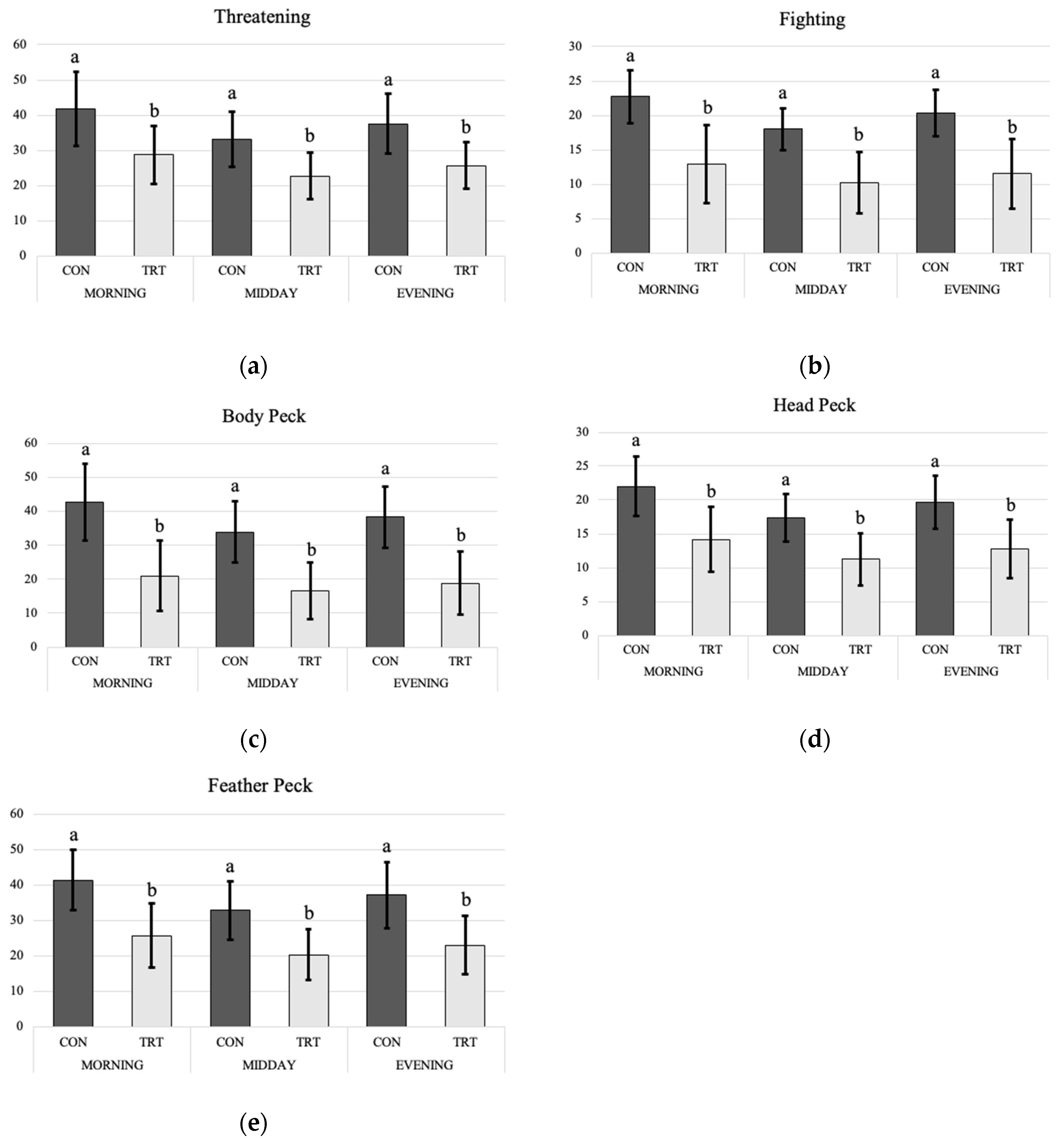
4.1. Behavior
4.2. Blood
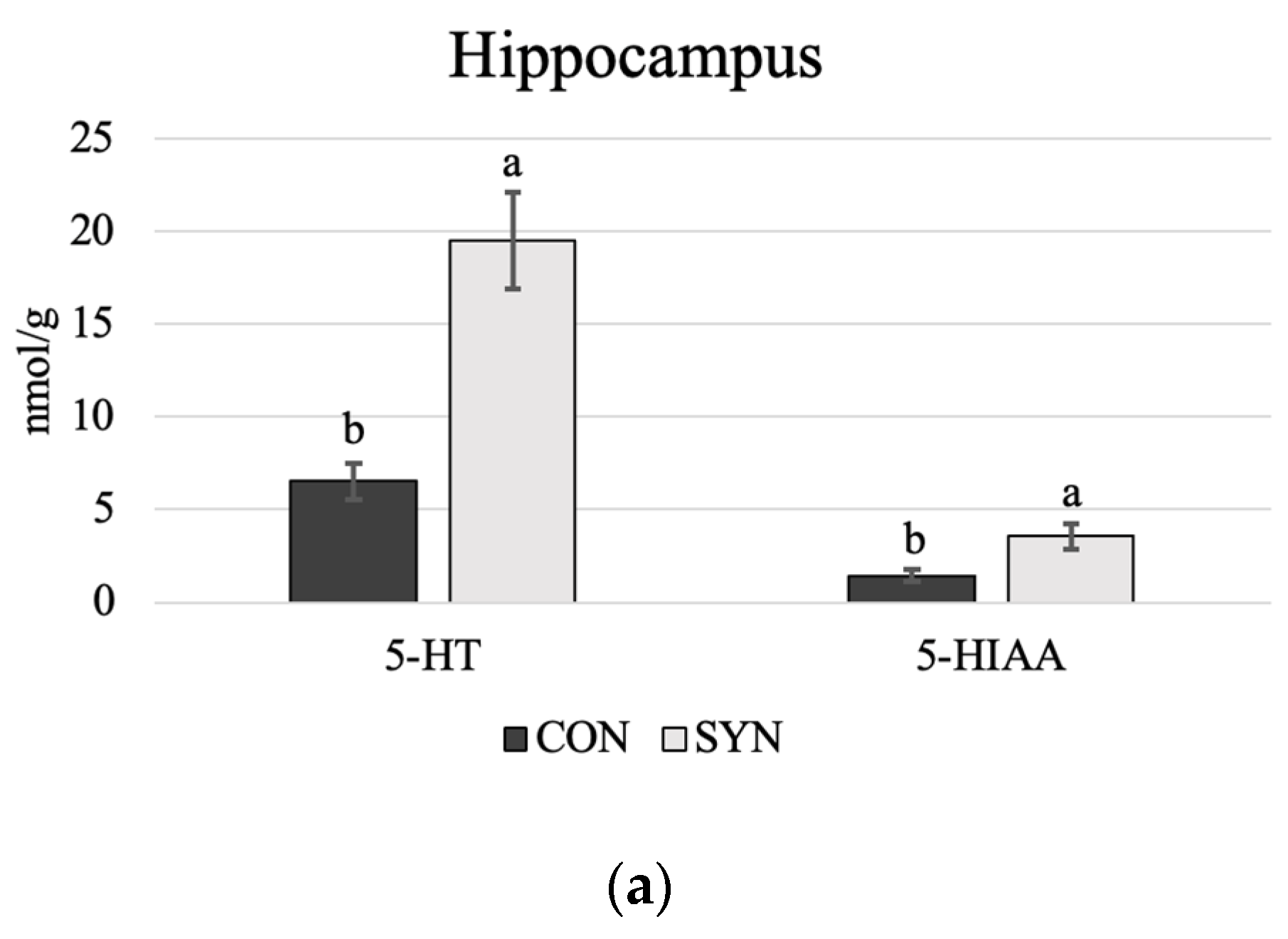
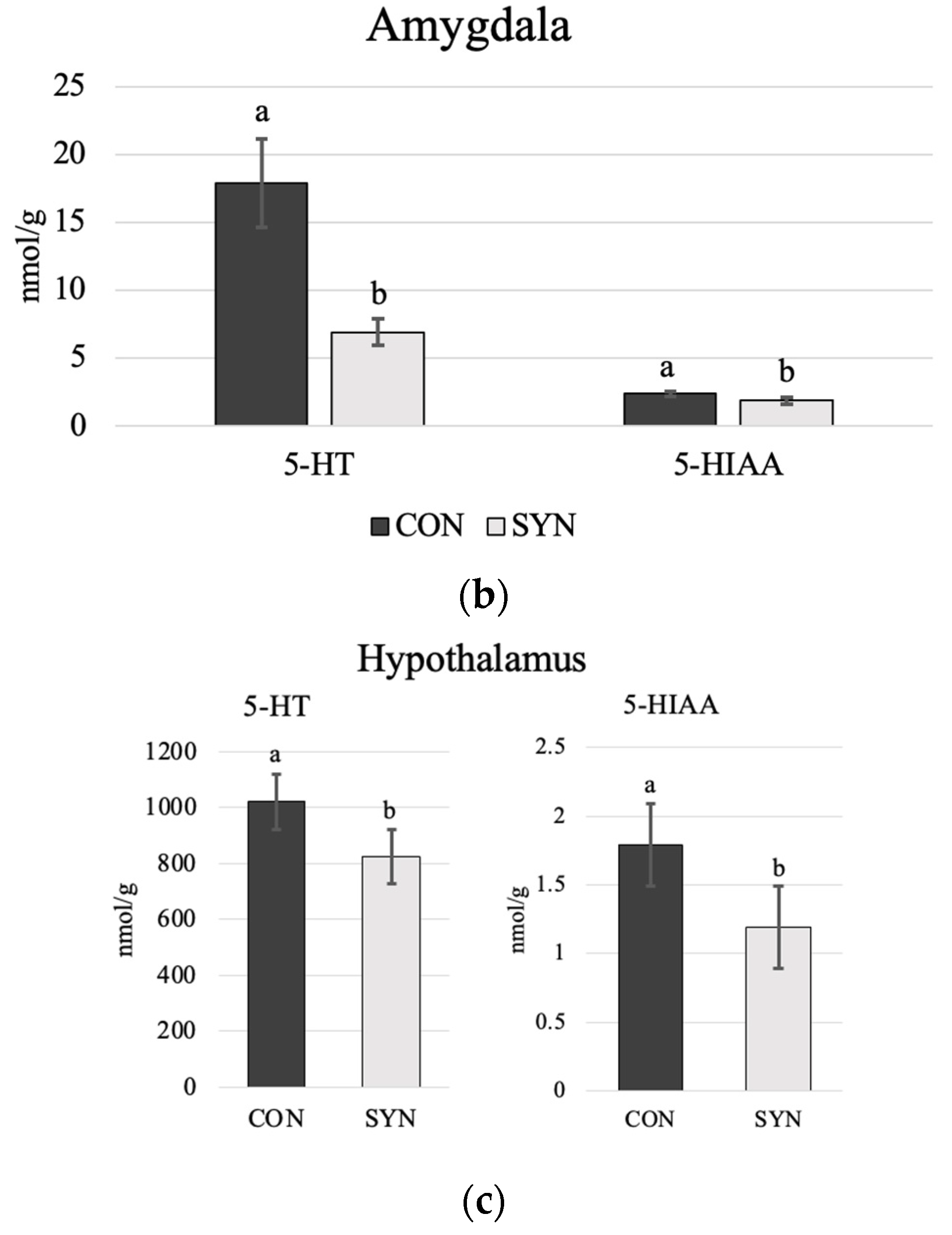
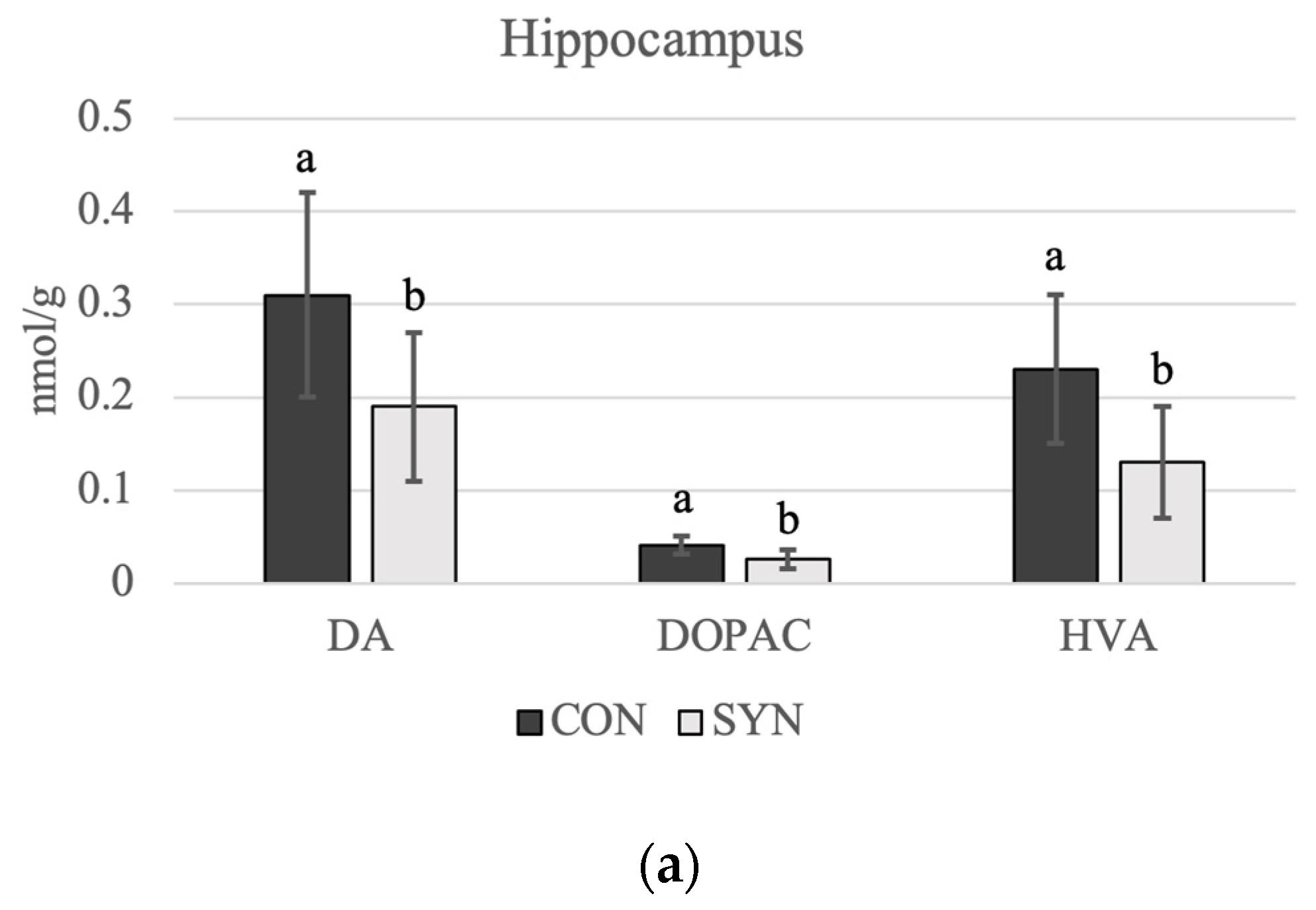
4.3. Brain Monoamines
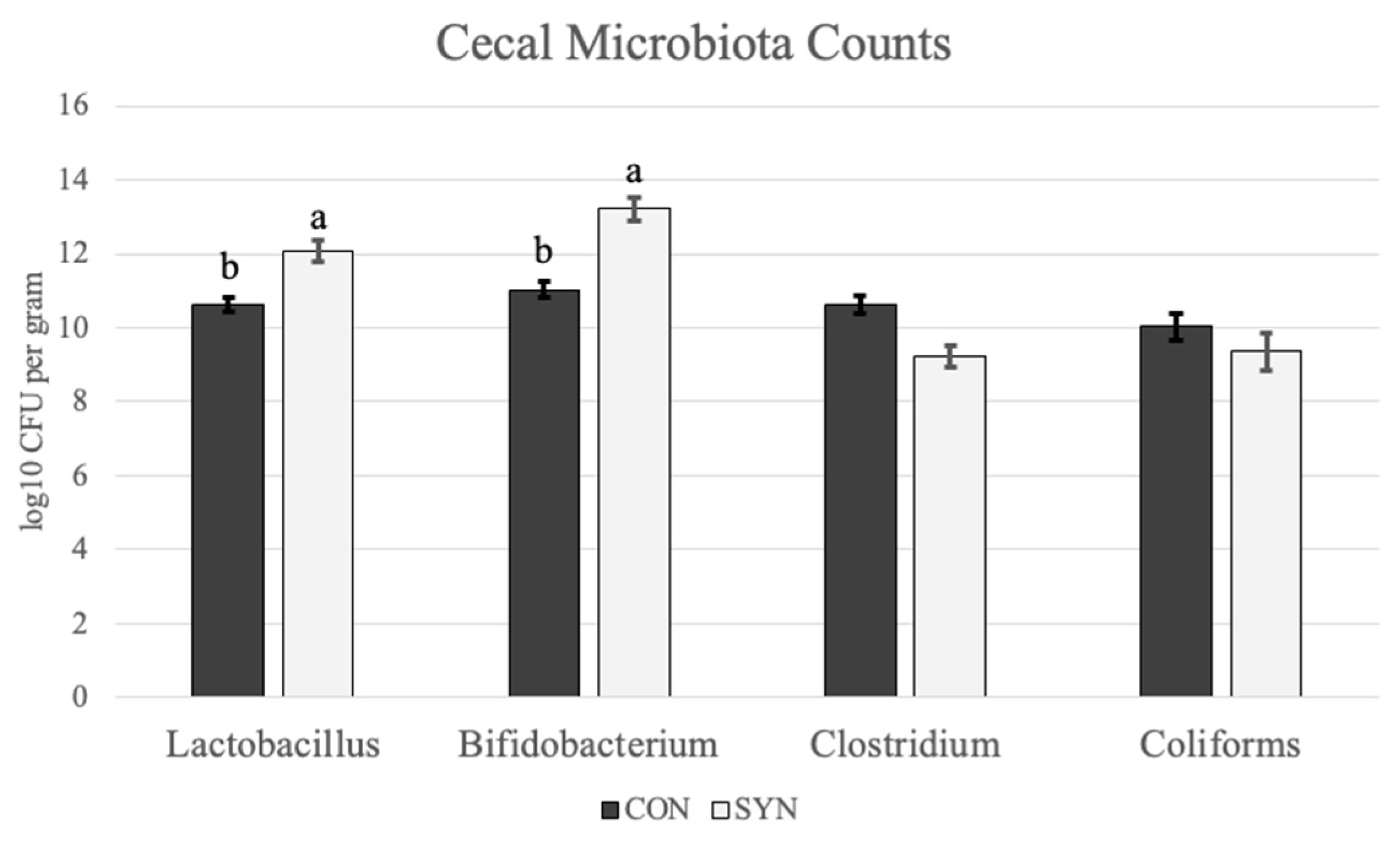
4.4. Cecal Microbiota Contents
5. Discussion
5.1. Behavior
5.2. Blood
5.3. Brain Monoamines
5.3.1. Serotonin
5.3.2. Dopamine
5.4. Cecal Microbiota
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the Microbiota-Gut-Brain Axis on Behavior and Welfare in Farm Animals: A Review. Physiol. Behav. 2019, 210, 112658. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Du, D.; Zhao, R.; Xie, Q.; Dong, Z. Gut Microbes Influence the Development of Central Nervous System Disorders through Epigenetic Inheritance. Microbiol. Res. 2023, 274, 127440. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential Feed Additives as Antibiotic Alternatives in Broiler Production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to Antibiotics for Organic Poultry Production: Types, Modes of Action and Impacts on Bird’s Health and Production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Naglaa; Azeem, M. Do Probiotics Affect the Behavior of Turkey Poults. J. Vet. Med. Anim. Health 2013, 5, 144–148. [Google Scholar]
- Yan, F.F.; Wang, W.C.; Cheng, H.W. Bacillus Subtilis Based Probiotic Improved Bone Mass and Altered Brain Serotoninergic and Dopaminergic Systems in Broiler Chickens. J. Funct. Foods 2018, 49, 501–509. [Google Scholar] [CrossRef]
- Huang, C.; Hao, E.; Yue, Q.; Liu, M.; Wang, D.; Chen, Y.; Shi, L.; Zeng, D.; Zhao, G.; Chen, H. Malfunctioned Inflammatory Response and Serotonin Metabolism at the Microbiota-Gut-Brain Axis Drive Feather Pecking Behavior in Laying Hens. Poult. Sci. 2023, 102, 102686. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. A Free, Versatile Open-source Event-logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Hughes, B.O.; Wood-Gush, D.G.M. Agonistic Behaviour in Domestic Hens: The Influence of Housing Method and Group Size. Anim. Behav. 1977, 25, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Mench, J.A. The Development of Aggressive Behavior in Male Broiler Chicks: A Comparison with Laying-Type Males and the Effects of Feed Restriction. Appl. Anim. Behav. Sci. 1988, 21, 233–242. [Google Scholar] [CrossRef]
- Girard, M.T.E.; Zuidhof, M.J.; Bench, C.J. Feeding, Foraging, and Feather Pecking Behaviours in Precision-Fed and Skip-a-Day-Fed Broiler Breeder Pullets. Appl. Anim. Behav. Sci. 2017, 188, 42–49. [Google Scholar] [CrossRef]
- Savory, C.J. Feather Pecking and Cannibalism. Worlds Poult. Sci. J. 1995, 51, 215–219. [Google Scholar] [CrossRef]
- Kops, M.S.; Kjaer, J.B.; Güntürkün, O.; Westphal, K.G.C.; Korte-Bouws, G.A.H.; Olivier, B.; Korte, S.M.; Bolhuis, J.E. Brain Monoamine Levels and Behaviour of Young and Adult Chickens Genetically Selected on Feather Pecking. Behav. Brain Res. 2017, 327, 11–20. [Google Scholar] [CrossRef] [PubMed]
- van Hierden, Y.M.; Korte, S.M.; Ruesink, E.W.; van Reenen, C.G.; Engel, B.; Korte-Bouws, G.A.H.; Koolhaas, J.M.; Blokhuis, H.J. Adrenocortical Reactivity and Central Serotonin and Dopamine Turnover in Young Chicks from a High and Low Feather-Pecking Line of Laying Hens. Physiol. Behav. 2002, 75, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kops, M.S.; de Haas, E.N.; Rodenburg, T.B.; Ellen, E.D.; Korte-Bouws, G.A.H.; Olivier, B.; Güntürkün, O.; Korte, S.M.; Bolhuis, J.E. Selection for Low Mortality in Laying Hens Affects Catecholamine Levels in the Arcopallium, a Brain Area Involved in Fear and Motor Regulation. Behav. Brain Res. 2013, 257, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Abdelqader, A.; Al-Fataftah, A.-R.; Daş, G. Effects of Dietary Bacillus Subtilis and Inulin Supplementation on Performance, Eggshell Quality, Intestinal Morphology and Microflora Composition of Laying Hens in the Late Phase of Production. Anim. Feed Sci. Technol. 2013, 179, 103–111. [Google Scholar] [CrossRef]
- Rushen, J. The Peck Orders of Chickens: How Do They Develop and Why Are They Linear? Anim. Behav. 1982, 30, 1129–1137. [Google Scholar] [CrossRef]
- Guhl, A.M. Social Inertia and Social Stability in Chickens. Anim. Behav. 1968, 16, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.C.; Blanchard, R.J. What Can Animal Aggression Research Tell Us about Human Aggression? Horm. Behav. 2003, 44, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sokołowicz, Z.; Dykiel, M.; Topczewska, J.; Krawczyk, J.; Augustyńska-Prejsnar, A. The Effect of the Type of Non-Caged Housing System, Genotype and Age on the Behaviour of Laying Hens. Animals 2020, 10, 2450. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Arentsen, T.; Raith, H.; Qian, Y.; Forssberg, H.; Heijtz, R.D. Host Microbiota Modulates Development of Social Preference in Mice. Microb. Ecol. Health Dis. 2015, 26, 29719. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.L.; Andretta, I.; Galli, G.M.; Martins, G.B.; Camargo, N.d.O.T.; Stefanello, T.B.; Melchior, R.; da Silva, M.K. Dietary Supplementation with β-Mannanase and Probiotics as a Strategy to Improve Laying Hen’s Welfare. Front. Vet. Sci. 2022, 9, 985947. [Google Scholar] [CrossRef] [PubMed]
- Tinbergen, N. On Aims and Methods of Ethology. Z. Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Dawkins, M.S. Natural Behaviour Is Not Enough: Farm Animal Welfare Needs Modern Answers to Tinbergen’s Four Questions. Animals 2023, 13, 988. [Google Scholar] [CrossRef]
- Cronin, G.M.; Hopcroft, R.L.; Groves, P.J.; Hall, E.J.S.; Phalen, D.N.; Hemsworth, P.H. Why Did Severe Feather Pecking and Cannibalism Outbreaks Occur? An Unintended Case Study While Investigating the Effects of Forage and Stress on Pullets during Rearing. Poult. Sci. 2018, 97, 1484–1502. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; Van Krimpen, M.M.; De Jong, I.C.; De Haas, E.N.; Kops, M.S.; Riedstra, B.J.; Nordquist, R.E.; Wagenaar, J.P.; Bestman, M.; Nicol, C.J. The Prevention and Control of Feather Pecking in Laying Hens: Identifying the Underlying Principles. Worlds Poult. Sci. J. 2013, 69, 361–374. [Google Scholar] [CrossRef]
- de Haas, E.N.; van der Eijk, J.A.J. Where in the Serotonergic System Does It Go Wrong? Unravelling the Route by Which the Serotonergic System Affects Feather Pecking in Chickens. Neurosci. Biobehav. Rev. 2018, 95, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Berk, J.; Bozakova, N.; van der Eijk, J.; Estevez, I.; Mircheva, T.; Relic, R.; Rodenburg, T.B.; Sossidou, E.N.; Guinebretière, M. The Relationships between Damaging Behaviours and Health in Laying Hens. Animals 2022, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Weeks, C.A.; Lambton, S.L.; Williams, A.G. Implications for Welfare, Productivity and Sustainability of the Variation in Reported Levels of Mortality for Laying Hen Flocks Kept in Different Housing Systems: A Meta-Analysis of Ten Studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef] [PubMed]
- Fossum, O.; Jansson, D.S.; Etterlin, P.E.; Vågsholm, I. Causes of Mortality in Laying Hens in Different Housing Systems in 2001 to 2004. Acta Vet. Scand. 2009, 51, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, J.; Yoon, H.-S.; Choi, Y.-H. Effects of Dietary Corticosterone on Yolk Colors and Eggshell Quality in Laying Hens. Asian-Australas. J. Anim. Sci. 2015, 28, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Mindus, C.; van Staaveren, N.; Fuchs, D.; Gostner, J.M.; Kjaer, J.B.; Kunze, W.; Mian, M.F.; Shoveller, A.K.; Forsythe, P.; Harlander-Matauschek, A.L. Rhamnosus Improves the Immune Response and Tryptophan Catabolism in Laying Hen Pullets. Sci. Rep. 2021, 11, 19538. [Google Scholar] [CrossRef]
- Linh, N.T.; Guntoro, B.; Qui, N.H. Immunomodulatory, Behavioral, and Nutritional Response of Tryptophan Application on Poultry. Vet. World 2021, 8, 2244–2250. [Google Scholar] [CrossRef]
- Koopmans, S.J.; van der Staay, F.J.; Le Floc’h, N.; Dekker, R.; van Diepen, J.T.M.; Jansman, A.J.M. Effects of Surplus Dietary L-Tryptophan on Stress, Immunology, Behavior, and Nitrogen Retention in Endotoxemic Pigs1. J. Anim. Sci. 2012, 90, 241–251. [Google Scholar] [CrossRef]
- van Hierden, Y.M.; Koolhaas, J.M.; Korte, S.M. Chronic Increase of Dietary L-Tryptophan Decreases Gentle Feather Pecking Behaviour. Appl. Anim. Behav. Sci. 2004, 89, 71–84. [Google Scholar] [CrossRef]
- Bello, A.U.; Idrus, Z.; Meng, G.Y.; Narayan, E.J.; Farjam, A.S. Dose-Response Relationship of Tryptophan with Large Neutral Amino Acids, and Its Impact on Physiological Responses in the Chick Model. Gen. Comp. Endocrinol. 2018, 260, 146–150. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the Essentials: Tryptophan Metabolism and the Microbiome-Gut-Brain Axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, J.; Erasmus, M.A.; Zhang, H.; Johnson, T.A.; Cheng, H. Cecal Microbiota Transplantation: Unique Influence of Cecal Microbiota from Divergently Selected Inbred Donor Lines on Cecal Microbial Profile, Serotonergic Activity, and Aggressive Behavior of Recipient Chickens. J. Anim. Sci. Biotechnol. 2023, 14, 66. [Google Scholar] [CrossRef]
- Reed, D. Enterochromaffin Cells: Small in Number but Big in Impact. Gastroenterology 2023, 165, 1090–1091. [Google Scholar] [CrossRef]
- Hu, J.; Johnson, T.A.; Zhang, H.; Cheng, H.-W. The Microbiota–Gut–Brain Axis: Gut Microbiota Modulates Conspecific Aggression in Diversely Selected Laying Hens. Microorganisms 2022, 10, 1081. [Google Scholar] [CrossRef]
- Jayamohananan, H.; Manoj Kumar, M.K.; Aneesh, T.P. 5-HIAA as a Potential Biological Marker for Neurological and Psychiatric Disorders. Adv. Pharm. Bull. 2019, 9, 374–381. [Google Scholar] [CrossRef]
- Zhou, M.; Tao, Y.; Lai, C.; Huang, C.; Zhou, Y.; Yong, Q. Effects of Mannanoligosaccharide Supplementation on the Growth Performance, Immunity, and Oxidative Status of Partridge Shank Chickens. Animals 2019, 9, 817. [Google Scholar] [CrossRef]
- Chenxuan, H.; Qiaoxian, Y.; Yifan, C.; Dehe, W.; Rongyan, Z.; Guoxian, Z.; Hui, C. Effects of in Ovo Injection of Serotonin on Behavior and Hypothalamic Genes Expression in Post Hatch-Chicks. Appl. Anim. Behav. Sci. 2021, 234, 105176. [Google Scholar] [CrossRef]
- Virkkunen, M.; Goldman, D.; Nielsen, D.A.; Linnoila, M. Low Brain Serotonin Turnover Rate (Low CSF 5-HIAA) and Impulsive Violence. J. Psychiatry Neurosci. 1995, 20, 271–275. [Google Scholar]
- Kops, M.S.; de Haas, E.N.; Rodenburg, T.B.; Ellen, E.D.; Korte-Bouws, G.A.H.; Olivier, B.; Güntürkün, O.; Bolhuis, J.E.; Korte, S.M. Effects of Feather Pecking Phenotype (Severe Feather Peckers, Victims and Non-Peckers) on Serotonergic and Dopaminergic Activity in Four Brain Areas of Laying Hens (Gallus Gallus Domesticus). Physiol. Behav. 2013, 120, 77–82. [Google Scholar] [CrossRef]
- Colombo, M.; Broadbent, N. Is the Avian Hippocampus a Functional Homologue of the Mammalian Hippocampus? Neurosci. Biobehav. Rev. 2000, 24, 465–484. [Google Scholar] [CrossRef]
- Fujita, T.; Aoki, N.; Mori, C.; Fujita, E.; Matsushima, T.; Homma, K.J.; Yamaguchi, S. Chick Hippocampal Formation Displays Subdivision- and Layer-Selective Expression Patterns of Serotonin Receptor Subfamily Genes. Front. Physiol. 2022, 13, 882633. [Google Scholar] [CrossRef]
- Dale, E.; Pehrson, A.L.; Jeyarajah, T.; Li, Y.; Leiser, S.C.; Smagin, G.; Olsen, C.K.; Sanchez, C. Effects of Serotonin in the Hippocampus: How SSRIs and Multimodal Antidepressants Might Regulate Pyramidal Cell Function. CNS Spectr. 2016, 21, 143–161. [Google Scholar] [CrossRef]
- Fujita, T.; Aoki, N.; Mori, C.; Homma, K.J.; Yamaguchi, S. Molecular Biology of Serotonergic Systems in Avian Brains. Front. Mol. Neurosci. 2023, 16, 1226645. [Google Scholar] [CrossRef]
- Fujita, T.; Aoki, N.; Mori, C.; Fujita, E.; Matsushima, T.; Homma, K.J.; Yamaguchi, S. The Dorsal Arcopallium of Chicks Displays the Expression of Orthologs of Mammalian Fear Related Serotonin Receptor Subfamily Genes. Sci. Rep. 2020, 10, 21183. [Google Scholar] [CrossRef]
- Reiner, A.; Perkel, D.J.; Bruce, L.L.; Butler, A.B.; Csillag, A.; Kuenzel, W.; Medina, L.; Paxinos, G.; Shimizu, T.; Striedter, G.; et al. Revised Nomenclature for Avian Telencephalon and Some Related Brainstem Nuclei. J. Comp. Neurol. 2004, 473, 377–414. [Google Scholar] [CrossRef]
- Saint-Dizier, H.; Constantin, P.; Davies, D.C.; Leterrier, C.; Lévy, F.; Richard, S. Subdivisions of the Arcopallium/Posterior Pallial Amygdala Complex Are Differentially Involved in the Control of Fear Behaviour in the Japanese Quail. Brain Res. Bull. 2009, 79, 288–295. [Google Scholar] [CrossRef]
- Spreux-Varoquaux, O.; Alvarez, J.-C.; Berlin, I.; Batista, G.; Despierre, P.-G.; Gilton, A.; Cremniter, D. Differential Abnormalities in Plasma 5-HIAA and Platelet Serotonin Concentrations in Violent Suicide Attempters Relationships with Impulsivity and Depression. Life Sci. 2001, 69, 647–657. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the Hypothalamic–Pituitary–Adrenal Function as a Tool to Evaluate Animal Welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Zhang, W.; Didehvar, D.; Wang, G.; Yi, J.; Gilbert, E.R.; Cline, M.A. Anorexigenic Effect of Serotonin Is Associated with Changes in Hypothalamic Nuclei Activity in an Avian Model. Gen. Comp. Endocrinol. 2017, 246, 81–87. [Google Scholar] [CrossRef]
- Walzem, R.L.; Chen, S. Obesity-Induced Dysfunctions in Female Reproduction: Lessons from Birds and Mammals. Adv. Nutr. 2014, 5, 199–206. [Google Scholar] [CrossRef]
- Beldowska, A.; Barszcz, M.; Dunislawska, A. State of the Art in Research on the Gut-Liver and Gut-Brain Axis in Poultry. J. Anim. Sci. Biotechnol. 2023, 14, 37. [Google Scholar] [CrossRef]
- Dennis, R.L.; Cheng, H.W. The Dopaminergic System and Aggression in Laying Hens. Poult. Sci. 2011, 90, 2440–2448. [Google Scholar] [CrossRef]
- McNamara, C.G.; Dupret, D. Two Sources of Dopamine for the Hippocampus. Trends Neurosci. 2017, 40, 383–384. [Google Scholar] [CrossRef]
- Kasperek, K.; Jaworska-Adamu, J.; Krawczyk, A.; Rycerz, K.; Buszewicz, G.; Przygodzka, D.; Wójcik, G.; Blicharska, E.; Drabik, K.; Czech, A.; et al. Investigation of Structural and Neurobiochemical Differences in Brains from High-Performance and Native Hen Breeds. Sci. Rep. 2023, 13, 224. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve Their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- D’Angio, M.; Serrano, A.; Driscoll, P.; Scatton, B. Stressful Environmental Stimuli Increase Extracellular DOPAC Levels in the Prefrontal Cortex of Hypoemotional (Roman High-Avoidance) but Not Hyperemotional (Roman Low-Avoidance) Rats. An in Vivo Voltammetric Study. Brain Res. 1988, 451, 237–247. [Google Scholar] [CrossRef]
- Tian, Y.; Eaton, M.J.; Goudreau, J.L.; Lookingland, K.J.; Moore, K.E. Neurochemical Evidence That 5-Hydroxytryptaminergic Neurons Tonically Inhibit Noradrenergic Neurons Terminating in the Hypothalamus. Brain Res. 1993, 607, 215–221. [Google Scholar] [CrossRef]
- Palermo-Neto, J. Dopaminergic Systems. Psychiatr. Clin. North Am. 1997, 20, 705–721. [Google Scholar] [CrossRef]
- Calefi, A.S.; Fonseca, J.G.d.S.; Nunes, C.A.d.Q.; Lima, A.P.N.; Quinteiro-Filho, W.M.; Flório, J.C.; Zager, A.; Ferreira, A.J.P.; Palermo-Neto, J. Heat Stress Modulates Brain Monoamines and Their Metabolites Production in Broiler Chickens Co-Infected with Clostridium perfringens Type A and Eimeria spp. Vet. Sci. 2019, 6, 4. [Google Scholar] [CrossRef]
- Bast, T.; Diekamp, B.; Thiel, C.; Schwarting, R.K.W.; Güntürkün, O. Functional Aspects of Dopamine Metabolism in the Putative Prefrontal Cortex Analogue and Striatum of Pigeons (Columba Livia). J. Comp. Neurol. 2002, 446, 58–67. [Google Scholar] [CrossRef]
- Yan, F.F.; Murugesan, G.R.; Cheng, H.W. Effects of Probiotic supplementation on Performance Traits, Bone Mineralization, Cecal Microbial Composition, Cytokines and Corticosterone in Laying Hens. Animal 2019, 13, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Quiroga, C.; Borda-Molina, D.; Chaves-Moreno, D.; Ruiz, R.; Atxaerandio, R.; Camarinha-Silva, A.; García-Rodríguez, A. Microbial and Functional Profile of the Ceca from Laying Hens Affected by Feeding Prebiotics, Probiotics, and Synbiotics. Microorganisms 2019, 7, 123. [Google Scholar] [CrossRef]
- Abdelqader, A.; Irshaid, R.; Al-Fataftah, A.-R. Effects of Dietary Probiotic Inclusion on Performance, Eggshell Quality, Cecal Microflora Composition, and Tibia Traits of Laying Hens in the Late Phase of Production. Trop. Anim. Health Prod. 2013, 45, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Lokapirnasari, W.P.; Pribadi, T.B.; Al Arif, A.; Soeharsono, S.; Hidanah, S.; Harijani, N.; Najwan, R.; Huda, K.; Wardhani, H.C.P.; Rahman, N.F.N.; et al. Potency of Probiotics Bifidobacterium spp. and Lactobacillus casei to Improve Growth Performance and Business Analysis in Organic Laying Hens. Vet. World 2019, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]

| Nutrient | Percentage |
|---|---|
| Crude protein (min.) | 16 |
| Lysine (min.) | 0.85 |
| Methionine (min.) | 0.36 |
| Crude fat (min.) | 3 |
| Crude fiber (max.) | 6.5 |
| Phosphorus (min.) | 0.6 |
| Calcium (min.–max.) | 4.1–4.4 |
| Salt (min.–max.) | 0.45–0.8 |
| Agonistic Behavior | Description |
|---|---|
| Head peck | A firm peck to the head with the receiver flinching |
| Fighting | Two hens actively jumping and pecking at one other. |
| Body peck | One hen using her beak to aggressively peck the body of a hen. |
| Threatening | Two hens in an upright, erect position with pecks delivered; the recipient often has an avoidance response |
| Feather peck | One hen aggressively pecking one bird and grabbing and/or pulling out feathers. |
| 50 Weeks | |||||
| CON | SYN | CON SEM | SYN SEM | p-Value | |
| CORT 1 | 36.52 | 39.26 | 4.52 | 3.69 | 0.230 |
| ACTH 2 | 13.88 | 15.58 | 1.36 | 2.06 | 0.152 |
| TRP 1 | 38.69 | 41.52 | 6.23 | 7.03 | 0.356 |
| DA 1 | 131.25 | 143.89 | 16.52 | 11.55 | 0.859 |
| 5-HT 1 | 142.36 | 139.55 | 9.69 | 12.85 | 0.659 |
| 5-HIAA 1 | 13.52 | 12.56 | 2.63 | 6.25 | 0.538 |
| 5-HT turnover | 0.11 | 0.13 | 0.06 | 0.09 | 0.693 |
| 60 Weeks | |||||
| CON | SYN | CON SEM | SYN SEM | p-Value | |
| CORT 1 | 42.36 a | 21.04 b | 1.56 | 1.11 | 0.026 |
| ACTH 2 | 15.96 a | 7.18 b | 22.47 | 11.98 | 0.006 |
| TRP 1 | 43.33 b | 70.96 a | 8.47 | 4.08 | 0.001 |
| DA 1 | 178.50 a | 114.41 b | 19.00 | 11.26 | 0.016 |
| 5-HT 1 | 172.26 a | 118.19 b | 10.95 | 11.25 | 0.003 |
| 5-HIAA 1 | 15.28 b | 26.17 a | 3.18 | 5.41 | 0.012 |
| 5-HT turnover | 0.13 b | 0.26 a | 0.09 | 0.08 | 0.001 |
| 5-HT | CON | SYN | CON SEM | SYN SEM | p-Value |
| Hippocampus | 0.24 a | 0.17 b | 0.09 | 0.08 | 0.001 |
| Amygdala | 0.12 b | 0.27 a | 0.03 | 0.08 | 0.026 |
| Hypothalamus | 0.011 a | 0.001 b | 0.001 | 0.001 | 0.032 |
| DA | CON | SYN | CON SEM | SYN SEM | p-Value |
| Hippocampus | 0.99 a | 0.81 b | 0.08 | 0.05 | 0.033 |
| Amygdala | 1.19 a | 1.01 b | 0.07 | 0.06 | 0.036 |
| Hypothalamus | 0.61 b | 0.79 a | 0.02 | 0.04 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.M.; Clark, A.; Anderson, M.G.; Corbin, E.; Arguelles-Ramos, M.; Ali, A.B.A. The Influence of Dietary Synbiotic on Agonistic Behavior, Stress, and Brain Monoamines via Modulation of the Microbiota–Gut–Brain Axis in Laying Hens. Poultry 2024, 3, 129-146. https://doi.org/10.3390/poultry3020011
Johnson AM, Clark A, Anderson MG, Corbin E, Arguelles-Ramos M, Ali ABA. The Influence of Dietary Synbiotic on Agonistic Behavior, Stress, and Brain Monoamines via Modulation of the Microbiota–Gut–Brain Axis in Laying Hens. Poultry. 2024; 3(2):129-146. https://doi.org/10.3390/poultry3020011
Chicago/Turabian StyleJohnson, Alexa M., Alexis Clark, Mallory G. Anderson, Elyse Corbin, Mireille Arguelles-Ramos, and Ahmed B. A. Ali. 2024. "The Influence of Dietary Synbiotic on Agonistic Behavior, Stress, and Brain Monoamines via Modulation of the Microbiota–Gut–Brain Axis in Laying Hens" Poultry 3, no. 2: 129-146. https://doi.org/10.3390/poultry3020011






