A Systematic In Silico Investigation of Phytochemicals from Artocarpus Species against Plasmodium falciparum Inhibitors †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Docking Simulations
2.2. In-Silico Drug-Likeness and ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) Analysis
3. Result and Discussion
3.1. Molecular Docking Simulations
3.2. In-Silico ADMET Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momčilović, S.; Cantacessi, C.; Arsić-Arsenijević, V.; Otranto, D.; Tasić-Otašević, S. Rapid diagnosis of parasitic diseases: Current scenario and future needs. Clin. Microbiol. Infect. 2019, 25, 290–309. [Google Scholar] [CrossRef] [PubMed]
- WHO Malaria Report. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 14 January 2022).
- Bouchut, A.; Rotili, D.; Pierrot, C.; Valente, S.; Lafitte, S.; Schultz, J.; Hoglund, U.; Mazzone, R.; Lucidi, A.; Fabrizi, G.; et al. Identification of novel quinazoline derivatives as potent antiplasmodial agents. Eur. J. Med. Chem. 2019, 161, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Benoit-Vical, F. Ethnomedicine in malaria treatment. IDrugs 2005, 8, 45–52. [Google Scholar] [PubMed]
- Shenai, B.R.; Sijwali, P.S.; Singh, A.; Rosenthal, P.J. Characterization of Native and Recombinant Falcipain-2, a Principal Trophozoite Cysteine Protease and Essential Hemoglobinase of plasmodium falciparum. J. Biol. Chem. 2000, 275, 29000–29010. [Google Scholar] [CrossRef] [PubMed]
- Sijwali, P.S.; Shenai, B.R.; Gut, J.; Singh, A.; Rosenthal, P.J. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biol. Chem. J. 2001, 360, 481–489. [Google Scholar] [CrossRef]
- Hogg, T.; Nagarajan, K.; Herzberg, S.; Chen, L.; Shen, X.; Jiang, H.; Wecke, M.; Blohmke, C.; Hilgenfeld, R.; Schmidt, C.L. Structural and functional characterization of Falcipain-2, a hemoglobinase from the malarial parasite Plasmodium falciparum. J. Biol. Chem. 2006, 281, 25425–25437. [Google Scholar] [CrossRef] [PubMed]
- Rajguru, T.; Bora, D.; Modi, M.K. Identification of promising inhibitors for Plasmodium haemoglobinase Falcipain-2, using virtual screening, molecular docking, and MD Simulation. J. Mol. Struct. 2022, 1248, 131427. [Google Scholar] [CrossRef]
- Lehmann, C.; Heitmann, A.; Mishra, S.; Burda, P.C.; Singer, M.; Prado, M.; Niklaus, L.; Lacroix, C.; Ménard, R.; Frischknecht, F.; et al. A cysteine protease inhibitor of plasmodium berghei is essential for exo-erythrocytic development. PLoS Pathog. 2014, 10, e1004336. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.M.; Maluf, S.E.C.; Azevedo, M.F.; Paschoalin, T.; Budu, A.; Bagnaresi, P.; Henrique-Silva, F.; Soares-Costa, A.; Gazarini, M.L.; Carmona, A.K. Inhibition of Plasmodium falciparum cysteine proteases by the sugarcane cystatin CaneCPI-4. Parasitol. Int. 2018, 67, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Fagnidi, Y.K.H.; Toi, B.; Megnassan, E.; Frecer, V.; Miertuš, S. In silico design of Plasmodium falciparum cysteine protease falcipain 2 inhibitors with favorable pharmacokinetic profile. J. Anal. Pharm. Res. 2018, 7, 298–309. [Google Scholar]
- Mali, S.N.; Pandey, A. Balanced QSAR and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against trypanosomiases. J. Comput. Biophys. Chem. 2022, 21, 83–114. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Molecular modeling studies on 2, 4-disubstituted imidazopyridines as anti-malarials: Atom-based 3D-QSAR, molecular docking, virtual screening, in-silico ADMET and theoretical analysis. J. Comput. Biophys. Chem. 2021, 20, 267–282. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A.; Thorat, B.R.; Lai, C.H. Multiple 3D-and 2D-quantitative structure–activity relationship models (QSAR), theoretical study and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against tuberculosis. Struct. Chem. 2022, 33, 679–694. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Unveiling Naturally Occurring Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCG) Targeting Mycobacterium DPRE1 for Anti-Tb Drug Discovery. Eng. Proc. 2021, 11, 31. [Google Scholar]
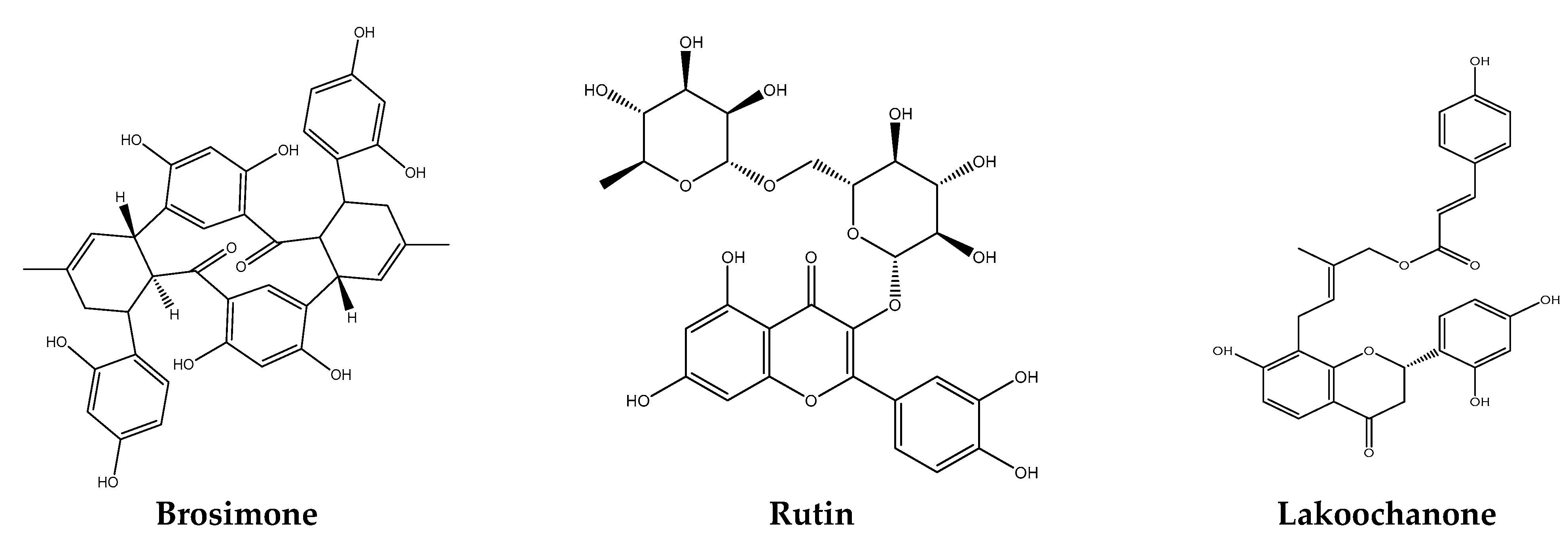
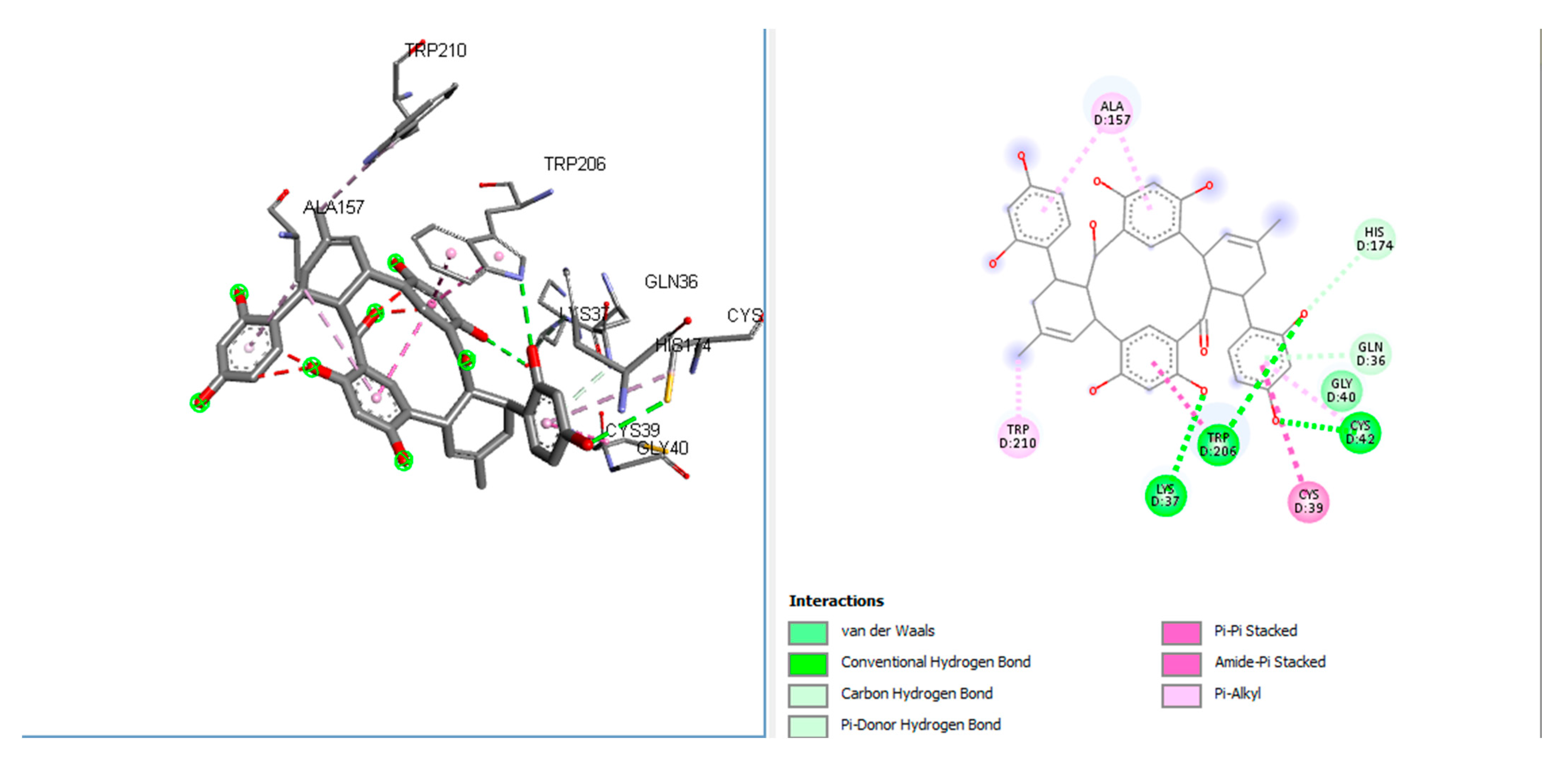
| Molecule | PyRx Interaction Energy |
|---|---|
| 1. Brosimone | −8.1 * |
| 2. Lakoochanone | −8 |
| 3. Rutin | −7.4 |
| Sr. No. | Molecules | Residues with Contribution Energy |
|---|---|---|
| 1. | Rutin | HIS D:19, GLU D:222, ALA A:157, TRP A:210, GLN A:209, LYS A:37, ASP A:35, CYS A:39 |
| 2. | Lakoochanone | GLY A:166, ASP A:155, LYS D:135, PHE A:164 |
| 3. | Brosimone | TRP D:210, LYS D:37, TRP D:206, CYS D:39, CYS D:42, GLY D:40, GLN D:40, HIS D:174, ALA D:157 |
| GI Absorption | BBB Permeant | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | Lipinski | Bioavailability Score | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Lakoochanone | Low | No | No | No | No | Yes | No | Yes | 0.55 |
| 2. Rutin | Low | No | Yes | No | No | No | No | No | 0.17 |
| 3. Brosimone | Low | No | Yes | No | No | No | No | No | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaurasia, S.; Pandey, A. A Systematic In Silico Investigation of Phytochemicals from Artocarpus Species against Plasmodium falciparum Inhibitors. Med. Sci. Forum 2022, 12, 5. https://doi.org/10.3390/eca2022-12712
Chaurasia S, Pandey A. A Systematic In Silico Investigation of Phytochemicals from Artocarpus Species against Plasmodium falciparum Inhibitors. Medical Sciences Forum. 2022; 12(1):5. https://doi.org/10.3390/eca2022-12712
Chicago/Turabian StyleChaurasia, Surabhi, and Anima Pandey. 2022. "A Systematic In Silico Investigation of Phytochemicals from Artocarpus Species against Plasmodium falciparum Inhibitors" Medical Sciences Forum 12, no. 1: 5. https://doi.org/10.3390/eca2022-12712






