Edible Paper Sheets from Alternanthera philoxeroides and Hypophthalmichthys molitrix: Smart Biomass Valorization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation of EPSs
2.2. Determination of Proximate Composition
2.3. Determination of Amino Acid Composition
2.4. Determination of Major Bioactive Compounds by GC-MS Analysis
2.5. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.6. Scanning Electron Microscope (SEM) and Energy-Dispersive X-ray (EDX) Analysis
2.7. Color Value Measurement of EPSs
2.8. Statistical Analyses of Experimental Data
3. Results and Discussion
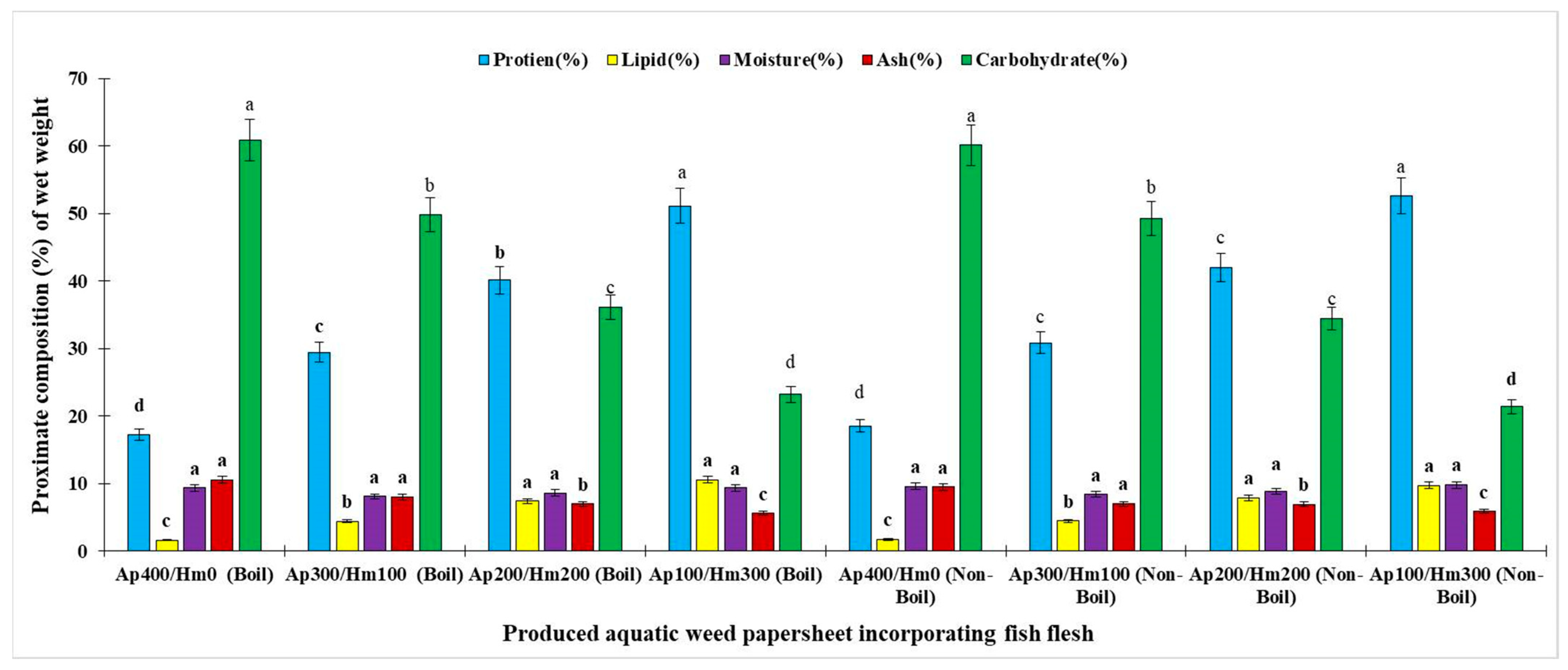
3.1. Proximate Composition of EPSs
3.1.1. Protein Content
3.1.2. Lipid Content
3.1.3. Moisture Content
3.1.4. Ash Content
3.1.5. Carbohydrate Content
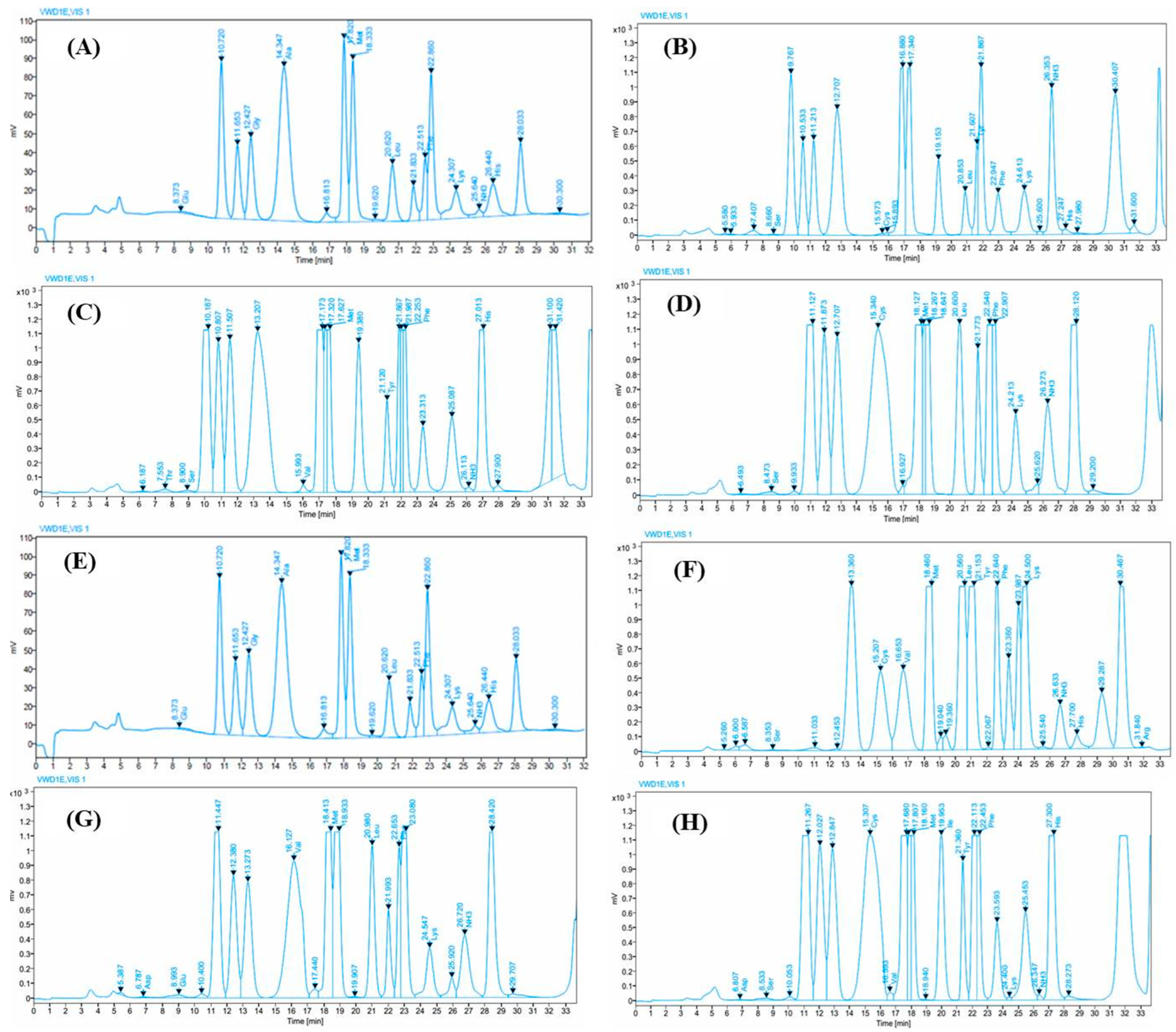
3.2. Amino Acid Composition of EPSs
3.3. Major Bioactive Compounds Available in EPS Detected by GC-MS Analysis
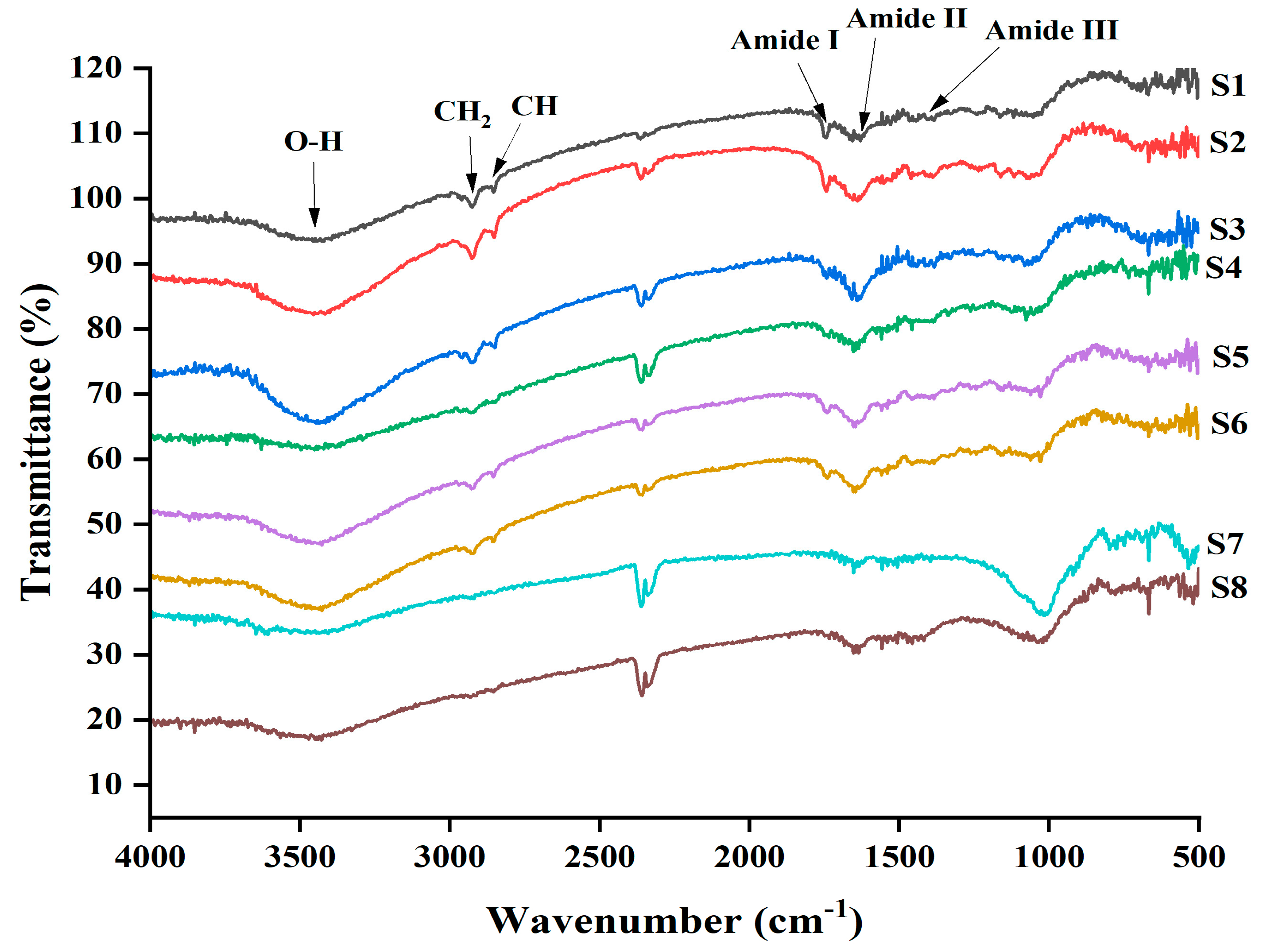
3.4. FT-IR Analysis of EPSs
3.5. SEM View and EDX Analysis of EPS
3.6. Color Values of EPSs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamsuzzaman, M.M.; Islam, M.M.; Tania, N.J.; Al-Mamun, M.A.; Barman, P.P.; Xu, X. Fisheries resources of Bangladesh: Present status and future direction. Aquacult. Fish. 2017, 2, 145–156. [Google Scholar] [CrossRef]
- Brouwer, P.; Nierop, K.G.; Huijgen, W.J.; Schluepmann, H. Aquatic weeds as novel protein sources: Alkaline extraction of tannin-rich Azolla. Biotechnol. Rep. 2019, 24, e00368. [Google Scholar] [CrossRef]
- Supawong, S.; Park, J.W.; Park, J. Functional and chemical properties of gim (Porphyra yezoensis) as affected by the product form. J. Aquat. Food Prod. Technol. 2022, 31, 418–429. [Google Scholar] [CrossRef]
- Sushil, K.; Kamlesh, V. Nutritive value of alligator weed [Alternanthera philoxeroides (Mart.) Griseb.] and its possible utility as a fodder in India. Ind. J. Weed Sci. 2005, 37, 152. [Google Scholar]
- Pamila, U.A.; Karpagam, S. A comparative study on phytoconsti-tuents of three different species of Genus Alternanthera. Int. J. Innov. Res. Adv. Stud. 2018, 5, 52–54. [Google Scholar]
- Suraiya, S.; Bristy, S.A.; Ali, M.S.; Biswas, A.; Ali, M.R.; Haq, M. A green approach to valorizing abundant aquatic weeds for nutrient-rich edible paper sheets production in Bangladesh. Clean Technol. 2023, 5, 1269–1286. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. An investigation into apricot pulp waste as a source of antioxidant polyphenols and carotenoid pigments. Biomass 2022, 2, 334–347. [Google Scholar] [CrossRef]
- Yuan, M.; Pickering, R.T.; Bradlee, M.L.; Mustafa, J.; Singer, M.R.; Moore, L.L. Animal protein intake reduces risk of functional impairment and strength loss in older adults. Clin. Nutr. 2021, 40, 919–927. [Google Scholar] [CrossRef]
- Desai, A.S.; Brennan, M.A.; Guo, X.; Zeng, X.A.; Brennan, C.S. Fish protein and lipid interactions on the digestibility and bioavailability of starch and protein from durum wheat pasta. Molecules 2019, 24, 839. [Google Scholar] [CrossRef]
- Cho, T.J.; Rhee, M.S. Health functionality and quality control of laver (Porphyra, Pyropia): Current issues and future perspectives as an edible seaweed. Mar. Drugs 2019, 18, 14. [Google Scholar] [CrossRef]
- Kabir, M.I.; Ove, T.A.; Kamal, M.M.; Karim, S.; Hasan, S.M.; Haque, M.R.; Akhter, M. Production and evaluation of quality characteristics of edible fish powder from tilapia (Oreochromis mossambicus) and silver carp (Hypophthalmichthys molitrix). J. Food Qual. 2022, 2022, 2530533. [Google Scholar] [CrossRef]
- Alavi Talab, H.; Ardjmand, M.; Motalebi, A.A.; Pourgholam, R. Optimization of morphology and geometry of encapsulated Hypophthalmichthys molitrix oil. Iran. J. Fish. Sci. 2010, 9, 199–208. [Google Scholar]
- Isra, L.; Suraiya, S.; Salma, U.; Haq, M. Nutritional evaluation and shelf-life study of mackerel tuna (Euthynnus affinis) fish pickle. Agr. Res. 2022, 11, 249–257. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Islam, M.A.; Mohibbullah, M.; Suraiya, S.; Sarower-E-Mahfuj, M.; Ahmed, S.; Haq, M. Nutritional characterization of freshwater mud eel (Monopterus cuchia) muscle cooked by different thermal processes. Food Sci. Nutr. 2020, 8, 6247–6258. [Google Scholar] [CrossRef]
- Jha, P.; Sudarshan, M.; Santra, S.C.; Dewanji, A. Elemental content in under-utilized green leafy vegetables of urban waterbodies in Kolkata, India and their associated health risk. J. Food Compos. Anal. 2023, 118, 105212. [Google Scholar] [CrossRef]
- Ullah, S.; Hasan, Z.; Zuberi, A.; Younus, N.; Rauf, S. Comparative study on body composition of two Chinese carps, common carp (Cyprinus carpio) and silver carp (Hypophthalmichthys molitrix). Glob. Vet. 2014, 13, 867–876. [Google Scholar]
- Hasan, M.M.; Nahar, S.; Arafat, S.T.; Debnath, S.; Parvez, M.S.; Rahman, S.M.; Ahsan, M.N. Proximate composition of edible aquatic vegetables: A preliminary assessment of four species from Bangladesh. Khulna Univ. Stud. 2016, 13, 49–53. [Google Scholar] [CrossRef]
- Dutta, P. Pharmacognostical evaluation and preliminary phytochemical analysis of Alternanthera philoxeroides. Int. J. Medi. Pharm. Res. 2015, 1, 7–13. [Google Scholar]
- Pulipati, S.; Devi, B.S.; Devi, G.R.; Bhanuja, M. Pharmacognostic studies of Alternanthera philoxeroides (mart.) griseb. J. Pharmacog. Phytochem. 2015, 4, 202–204. [Google Scholar]
- Harper, J.M. Ash content determination in foods: A review of current techniques and applications. Food Chem. 2016, 197, 1280–1287. [Google Scholar]
- Satter, M.M.A.; Khan, M.M.R.L.; Jabin, S.A.; Abedin, N.; Islam, M.F.; Shaha, B. Nutritional quality and safety aspects of wild vegetables consume in Bangladesh. Asian Pac. J. Trop. Biomed. 2016, 6, 125–131. [Google Scholar] [CrossRef]
- Umar, K.J.; Hassan, L.G.; Dangoggo, S.M.; Ladan, M.J. Nutritional composition of water spinach (Ipomoea aquatica Forsk.) leaves. J. Appl. Sci. 2007, 7, 803–809. [Google Scholar] [CrossRef]
- Dewanji, A. Amino acid composition of leaf proteins extracted from some aquatic weeds. J. Agric. Food Chem. 2024, 41, 1232–1236. [Google Scholar] [CrossRef]
- Sabetian, M.; Delshad, S.T.; Moini, S.; Islami, H.R.; Motalebi, A. Identification of fatty acid content, amino acid profile and proximate composition in rainbow trout (Oncorhynchus mykiss). J. Am. Sci. 2012, 8, 670–677. [Google Scholar]
- Aon-im, P.; Monthakantirat, O.; Daodee, S.; Chulikhit, Y.; Sriya, N.; Boonyarat, C.; Chumwangwapee, T.; Khamphukdee, C.; Kijjoa, A. Evaluation of the impact of Alternanthera philoxeroides (Mart.) Griseb. Extract on memory impairment in D-galactose-induced brain aging in mice through its effects on antioxidant enzymes, neuroinflammation, and telomere shortening. Molecules 2024, 29, 503. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Amin, A.; Khalil, T.; Iqbal, M.S.; Nazir, A.; Taswar, A. Antibacterial activity of Alternanthera philoxeroides (Mart.) Griseb. against bacterial phytopathogens: Erwinia carotovora, Ralstonia solanacearum and Xanthomonas axonopodis. Allelopath. J. 2021, 53, 83–92. [Google Scholar] [CrossRef]
- Song, W.; Ding, L.; Liu, M.; Cheng, J.; Zhou, J.; Li, Y.Y. Improving biohydrogen production through dark fermentation of steam-heated acid pretreated Alternanthera philoxeroides by mutant Enterobacter aerogenes ZJU1. Sci. Total Environ. 2020, 716, 134695. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, F.; Zhao, M.; Yang, B.; Cui, C. Physicochemical changes of myofibrillar proteins during processing of Cantonese sausage in relation to their aggregation behaviour and in vitro digestibility. Food Chem. 2011, 129, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Sanden, K.W.; Kohler, A.; Afseth, N.K.; Böcker, U.; Rønning, S.B.; Liland, K.H.; Pedersen, M.E. The use of Fourier-transform infrared spectroscopy to characterize connective tissue components in skeletal muscle of Atlantic cod (Gadus morhua L.). J. Biophotonics 2019, 12, e201800436. [Google Scholar] [CrossRef]
- Careche, M.; Carmona, P.; Sánchez-Alonso, I. Monitoring the time and temperature history of frozen hake (Merluccius merluccius L.) muscle by FTIR spectroscopy of the lipid fraction. Food Bioprocess Technol. 2015, 8, 112–119. [Google Scholar] [CrossRef]
- Ural, N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: An overview. Open Geosci. 2021, 13, 197–218. [Google Scholar] [CrossRef]


| Sample Tags | A. philoxeroides (g) | H. molitrix (g) | Normal Salt (g) | Water (mL) | Technique |
|---|---|---|---|---|---|
| Ap400/Hm0 (Boil) | 400 | 0 | 2 | 200 | Boil |
| Ap300/Hm100 (Boil) | 300 | 100 | 2 | 200 | Boil |
| Ap200/Hm200 (Boil) | 200 | 200 | 2 | 200 | Boil |
| Ap100/Hm300 (Boil) | 100 | 300 | 2 | 200 | Boil |
| Ap400/Hm0 (Non-boil) | 400 | 0 | 2 | 200 | Non-boil |
| Ap300/Hm100 (Non-boil) | 300 | 100 | 2 | 200 | Non-boil |
| Ap200/Hm200 (Non-boil) | 200 | 200 | 2 | 200 | Non-boil |
| Ap100/Hm300 (Non-boil) | 100 | 300 | 2 | 200 | Non-boil |
| Amino Acids | Ap400/Hm0 (Boil) | Ap300/Hm100 (Boil) | Ap200/Hm200 (Boil) | Ap100/Hm300 (Boil) | Ap400/Hm0 (Non-Boil) | Ap300/Hm100 (Non-Boil) | Ap200/Hm200 (Non-Boil) | Ap100/Hm300 (Non-Boil) |
|---|---|---|---|---|---|---|---|---|
| Ser | n.d. | 88.31 ± 2.24 c | 179.02 ± 5.85 b | 298.33 ± 4.65 a | n.d. | 97.11 ± 2.54 c | 191.87 ± 6.25 b | 286.68 ± 5.50 a |
| Cys | n.d. | 126.80 ± 5.58 c | 728.14 ± 12.25 b | 1544.88 ± 18.25 a | n.d. | 113.65 ± 5.25 c | 750.52 ± 10.25 b | 1532.69 ± 20.25 a |
| Met | 804.77 ± 14.25 c | 465.24 ± 5.25 d | 6666.62 ± 75.25 b | 11,990.94 ± 780.25 a | 901.55 ± 18.54 c | 458.33 ± 5.57 d | 6680.19 ± 60.25 b | 12,161.79 ± 87.25 a |
| Leu | 2072.24 ± 45.52 d | 2705.27 ± 50.25 c | 4348.96 ± 98.22 b | 4938.94 ± 140.25 a | 2194.50 ± 85.22 d | 2648.83 ± 75.25 c | 4397.27 ± 83.25 b | 4905.93 ± 90.25 a |
| Phe | 3394.24 ± 45.67 c | 3067.87 ± 48.25 d | 4138.08 ± 73.25 b | 4677.47 ± 82.25 a | 3693.12 ± 76.35 c | 2904.41 ± 49.25 d | 3975.66 ± 80.25 b | 4762.60 ± 92.25 a |
| Lys | 4270.07 ± 140.25 d | 4768.48 ± 87.01 c | 5476.14 ± 58.47 b | 4640.63 ± 76.35 a | 4294.84 ± 82.25 d | 4676.02 ± 47.58 c | 5543.00 ± 90.25 b | 4604.12 ± 45.36 a |
| Thr | n.d. | 203.60 ± 12.21 c | 314.19 ± 14.20 b | 376.79 ± 5.25 a | n.d. | 192.70 ± 4.58 c | 304.07 ± 8.52 b | 350.90 ± 9.35 a |
| Val | 408.17 ± 10.25 d | 3278.15 ± 98.25 c | 4054.30 ± 80.35 b | 4455.23 ± 48.25 a | 410.14 ± 10.24 d | 3114.36 ± 57.36 c | 4111.46 ± 83.25 b | 4204.43 ± 86.25 a |
| Tyr | 2399.43 ± 45.25 d | 2704.87 ± 75.28 c | 4274.41 ± 46.45 b | 4746.36 ± 46.25 a | 2555.32 ± 74.18 c | 2630.74 ± 80.29 c | 4278.56 ± 46.80 b | 4498.97 ± 47.58 a |
| His | 408.17 ± 12.25 d | 442.47 ± 10.27 c | 1105.99 ± 8.25 b | 1511.63 ± 17.25 a | 455.50 ± 18.34 d | 3819.45 ± 20.27 c | 1192.77 ± 42.25 b | 1680.86 ± 41.73 a |
| Asp | 242.92 ± 10.27 d | 5619.94 ± 44.25 c | 762.22 ± 8.25 b | 975.81 ± 6.34 a | 240.03 ± 4.35 d | 4447.39 ± 4.58 c | 763.72 ± 8.54 b | 1169.06 ± 8.25 a |
| Sl No | Identifying Compound | Molecular Weight | Molecular Formula | Peak Area (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ap400/Hm0 (Boil) | Ap300/Hm100 (Boil) | Ap200/Hm200 (Boil) | Ap100/Hm300 (Boil) | Ap400/Hm0 (Non-Boil) | Ap300/Hm100 (Non-Boil) | Ap200/Hm200 (Non-Boil) | Ap100/Hm300 (Non-Boil) | ||||
| 1 | Carbonic acid, 2-dimethylaminoethyl ethyl ester | 161 | C7H15NO3 | 1.54 | 1.42 | 2.04 | 0.02 | 0.56 | 0.53 | 1.77 | 0.25 |
| 2 | Acetamide, 2-amino-n-ethyl-2-thioxo- | 132 | C4H8N2OS | - | - | 0.03 | - | - | 0.09 | - | - |
| 3 | Propane, 2-chloro-2-nitro- | 123 | C3H6ClNO2 | - | - | 0.15 | - | - | - | 0.49 | - |
| 4 | Propamocarb | 188 | C9H20N2O2 | - | - | 0.45 | - | 0.28 | 0.21 | 0.49 | - |
| 5 | Oxime-, methoxy-phenyl-_ | 151 | C8H9NO2 | - | 0.53 | 1.24 | 2.99 | - | 0.20 | 1.33 | 2.14 |
| 6 | Oxime-, methoxy-phenyl-_ | 151 | C8H9NO2 | - | 0.53 | - | 2.99 | - | 0.20 | 0.33 | - |
| 7 | D-Methionine | 149 | C5H11NO2S | 3.22 | 1.25 | 0.21 | - | - | 3.25 | 2.40 | 1.19 |
| 8 | Phthalan | 120 | C8H8O | - | - | 1.07 | 1.12 | - | 2.82 | - | - |
| 9 | Dimethylisopropylsilyloxycyclobutane | 172 | C9H20OSi | 1.45 | 0.25 | - | - | - | 1.25 | - | - |
| 10 | Tetrazolo[5,1-a]phthalazin-6-amine, n-methyl- | 200 | C9H8N6 | - | 2.25 | - | -- | - | - | 0.37 | - |
| 11 | Diethyl 1-methyl-3-hydroxy-5-phenylpyrrole-2,4-dicarboxylate | 317 | C17H19NO5 | - | - | - | 3.45 | 0.31 | 0.37 | 0.59 | 2.21 |
| 12 | Hentriacontane | 436 | C31H64 | - | 0.51 | - | - | 0.13 | 0.20 | - | - |
| 13 | Hexadecane | 226 | C16H34 | - | 0.46 | 0.50 | - | 0.18 | 0.26 | 0.31 | - |
| 14 | Hentriacontane | 436 | C31H64 | - | 0.51 | 0.44 | 0.98 | 0.13 | 0.20 | 0.25 | - |
| 15 | Hentriacontane | 436 | C31H64 | - | 0.51 | 0.44 | 0.98 | 0.13 | 0.20 | 0.25 | - |
| 16 | S-butyl n-hexyl disulfide | 206 | C10H22S2 | - | 1.87 | 0.50 | - | 0.72 | 0.54 | - | - |
| 17 | Phosphoric acid, bis(trimethylsilyl)monomethyl ester | 256 | C7H21O4PSi2 | 39.54 | 35.02 | 29.88 | 20.40 | 37.64 | 31.38 | 28.33 | 19.25 |
| 18 | Triacontane | 422 | C30H62 | - | - | - | 0.71 | 0.32 | - | 0.36 | - |
| 19 | Hexadecane | 226 | C16H34 | - | 0.46 | 0.50 | - | 0.18 | 0.26 | 0.31 | - |
| 20 | Pipradrol | 267 | C18H21NO | 0.88 | - | - | - | - | - | - | 1.46 |
| 21 | 2,4-difluorobenzoic acid, 2-formyl-4,6-dichlorophenyl ester | 330 | C14H6Cl2F2O3 | - | - | 0.5 | 1.24 | 0.17 | 0.16 | - | 1.05 |
| 22 | Dodecanoic acid | 200 | C9H8N6 | 2.50 | 2.25 | 1.58 | -- | 2.47 | 2.14 | 1.37 | - |
| 23 | Tritetracontane | 604 | C43H88 | - | - | - | - | - | 0.26 | - | - |
| 24 | Triacontane | 422 | C30H62 | - | - | - | - | 0.32 | - | 0.36 | - |
| 25 | Quinoline, 2-Ethyl- | 157 | C11H11N | 0.27 | - | - | - | - | - | - | 0.19 |
| 26 | Dl-Alanyl-Dl-Alanine | 482 | C17H28N2O5 | - | - | - | - | - | - | 0.16 | |
| 27 | Oleic acid | 282 | C18H34O2 | 21.01 | 20.41 | 15.00 | 10.42 | 21.83 | 12.20 | 10.25 | 5.34 |
| 28 | N-decanoic acid | 172 | C10H20O2 | - | 0.98 | - | - | 5.10 | 1.34 | 0.31 | - |
| 29 | Isoheptadecanol | 256 | C17H36O | 28.24 | 24.24 | 21.88 | 11.83 | 37.64 | 39.38 | 38.33 | 24.02 |
| 30 | Methyl 11-Methyl-Dodecanoate | 228 | C14H28O2 | 4.46 | 3.25 | 2.14 | 1.24 | 3.54 | 2.47 | 1.43 | 2.54 |
| 31 | Undecanoic Acid,10-Methyl-,Methyl Ester | 214 | C13H26O2 | 0.05 | - | - | - | - | - | - | 8.05 |
| 32 | Tetradecanoic acid, 10,13-dimethyl-, methyl ester | 270 | C17H34O2 | 2.14 | 2.36 | 3.50 | 4.80 | 1.31 | 1.30 | 1.91 | 2.14 |
| 33 | N-hexadecanoic acid | 256 | C16H32O2 | 2.14 | 5.24 | 9.88 | 0.40 | 7.64 | 9.38 | 8.33 | 10.25 |
| 34 | Phytol | 296 | C20H40O | 2.89 | - | - | - | - | - | - | 14.02 |
| 35 | 13-octadecenoic acid, methyl ester | 296 | C19H36O2 | - | - | - | 0.50 | 2.15 | - | - | - |
| 36 | Heptadecanoic acid, 16-methyl-, methyl ester | 298 | C19H38O2 | - | - | - | 0.03 | 0.08 | - | - | - |
| 37 | Methyl 8,11,14-heptadecatrienoate | 278 | C18H30O2 | 0.56 | 1.43 | 1.33 | 1.14 | - | 1.00 | 1.43 | 1.51 |
| 38 | Ethyl 9,12,15-octadecatrienoate | 306 | C20H34O2 | - | - | - | - | 0.56 | 0.74 | - | - |
| 39 | 11,14,17-Eicosatrienoic Acid, Methyl Ester | 320 | C21H36O2 | 6.33 | 12.52 | 13.54 | 15.27 | 5.25 | 11.24 | 13.25 | 13.81 |
| 40 | N-propyl 11-octadecenoate | 324 | C21H40O2 | 0.25 | 3.79 | 5.25 | 6.96 | 0.12 | 8.25 | 10.81 | 12.47 |
| 41 | Pentadecanoic acid, 14-bromo- | 320 | C15H29BrO2 | 2.14 | 5.25 | 11.24 | 15.58 | 1.24 | 4.25 | 10.70 | 14.27 |
| 42 | N-propyl 11-octadecenoate | 324 | C21H40O2 | - | 3.79 | 11.47 | 15.24 | - | 2.25 | 5.81 | 7.5 |
| 43 | 3-cyclopentylpropionic acid, 2-dimethylaminoethyl ester | 213 | C12H23NO2 | 2.74 | 8.12 | 4.22 | 5.86 | - | 4.04 | - | - |
| 44 | Cis-2-methyl-4-n-butylthiane, s,s-dioxide | 204 | C10H20O2S | - | - | - | 1.65 | - | 1.95 | - | - |
| 45 | Glycidyl palmitate | 312 | C19H36O3 | - | 1.33 | 1.42 | 2.84 | 1.94 | 2.13 | 2.85 | 3.25 |
| 46 | Glycidyl palmitate | 312 | C19H36O3 | - | 1.33 | - | 6.84 | 1.94 | - | 2.85 | - |
| 47 | 2-amino-4-methyl-6-(2-thienyl)pyrimidine | 191 | C9H9N3S | - | - | - | 5.47 | - | - | 0.73 | - |
| 48 | Phenol, 2,5-bis(1,1-dimethylethyl)- | 206 | C10H22S2 | - | 1.87 | 0.50 | - | 0.72 | 0.54 | - | - |
| 49 | 1,2,3,4-tetrahydro-3-(phenylacetamido)quinoline | 266 | C17H18N2O | 9.28 | 8.95 | 7.22 | 5.25 | 10.56 | 8.32 | 6.28 | 4.25 |
| 50 | 1,2,3,4-tetrahydro-3-(phenylacetamido)quinoline | 266 | C17H18N2O | - | 2.14 | 1.85 | 1.64 | 2.63 | 2.32 | 1.28 | 0.76 |
| 51 | Acetic acid, (dodecahydro-7-hydroxy-1,4b,8,8-tetramethyl-10-oxo-2(1h) | 405 | C24H39NO4 | - | 1.46 | 1.74 | - | - | - | - | - |
| Serial | Samples | Lightness (L*) | Red to Green (a*) | Yellow to Blue (b*) |
|---|---|---|---|---|
| 1. | Ap400/Hm0 (Boil) | 24.37 ± 1.25 d | 15.40 ± 0.58 a | 9.24 ± 0.47 a |
| 2. | Ap300/Hm100 (Boil) | 25.83 ± 0.85 c | 9.25 ± 0.10 c | 4.77 ± 0.54 b |
| 3. | Ap200/Hm200 (Boil) | 28.28 ± 1.23 b | 7.14 ± 0.76 d | 3.62 ± 0.37 c |
| 4. | Ap100/Hm300 (Boil) | 30.97 ± 1.70 a | 5.20 ± 0.47 d | 3.63 ± 0.74 c |
| 5. | Ap400/Hm0 (Non-boil) | 25.95 ± 0.76 d | 16.70 ± 0.43 a | 10.12 ± 0.18 a |
| 6. | Ap300/Hm100 (Non-boil) | 26.43 ± 1.81 c | 13.25 ± 1.07 b | 4.52 ± 0.40 b |
| 7. | Ap200/Hm200 (Non-boil) | 27.38 ± 1.05 b | 9.60 ± 1.04 c | 3.70 ± 0.20 c |
| 8. | Ap100/Hm300 (Non-boil) | 29.47 ± 1.73 a | 5.24 ± 0.74 d | 3.47 ± 0.14 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suraiya, S.; Mohona, M.A.S.; Fatema, M.; Haq, M.; Rahman, M.A.; Mondal, S. Edible Paper Sheets from Alternanthera philoxeroides and Hypophthalmichthys molitrix: Smart Biomass Valorization. Biomass 2024, 4, 414-428. https://doi.org/10.3390/biomass4020020
Suraiya S, Mohona MAS, Fatema M, Haq M, Rahman MA, Mondal S. Edible Paper Sheets from Alternanthera philoxeroides and Hypophthalmichthys molitrix: Smart Biomass Valorization. Biomass. 2024; 4(2):414-428. https://doi.org/10.3390/biomass4020020
Chicago/Turabian StyleSuraiya, Sharmin, Mst. Ayesha Siddika Mohona, Mst Fatema, Monjurul Haq, Md. Anisur Rahman, and Subrata Mondal. 2024. "Edible Paper Sheets from Alternanthera philoxeroides and Hypophthalmichthys molitrix: Smart Biomass Valorization" Biomass 4, no. 2: 414-428. https://doi.org/10.3390/biomass4020020







