Dose-Dependency of the Glycemic Response to Polyphenol-Rich Sugarcane Extract (PRSE)
Abstract
:1. Introduction
2. Results
2.1. Postprandial Glucose Measurement of One Dose of Sugar Sample
2.2. Trial Extension: Additional Dosages
2.3. Glycemic Index
3. Discussion
3.1. Postprandial Glucose Measurement of Sugar Samples
3.2. Glycemic Index
4. Materials and Methods
4.1. Preparation of PRSE
4.2. Determination of the Polyphenol Content in the PRSE
4.3. Determination of Glycemic Index
4.4. Test Subjects
4.5. Test Foods
4.6. Measurement of Plasma Glucose Concentrations and GI Values
4.7. Statistical Analysis
4.8. Experimental Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, M.; Nazareth, I.; Petersen, I. Trends in Incidence, Prevalence and Prescribing in Type 2 Diabetes Mellitus between 2000 and 2013 in Primary Care: A Retrospective Cohort Study. BMJ Open 2016, 6, e010210. [Google Scholar] [CrossRef] [PubMed]
- Zghebi, S.S.; Steinke, D.T.; Carr, M.J.; Rutter, M.K.; Emsley, R.A.; Ashcroft, D.M. Examining Trends in Type 2 Diabetes Incidence, Prevalence and Mortality in the UK between 2004 and 2014. Diabetes Obes. Metab. 2017, 19, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, M.L.; Eriksen, N.M.; Mortensen, L.S.; Holm, L.; Hermansen, K. Can the Glycemic Index (GI) Be Used as a Tool in the Prevention and Management of Type 2 Diabetes? Rev. Diabet. Stud. 2006, 3, 61–71. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update); Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Stanhope, K.L. Sugar Consumption, Metabolic Disease and Obesity: The State of the Controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Stockmann, K.; Atkinson, F.; Petocz, P.; Denyer, G. Glycemic Index, Postprandial Glycemia, and the Shape of the Curve in Healthy Subjects: Analysis of a Database of More than 1000 Foods. Am. J. Clin. Nutr. 2009, 89, 97–105. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic Index, Glycemic Load and Glycemic Response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory Effects of Polyphenols on Apoptosis Induction: Relevance for Cancer Prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Guo, W.; Kong, E.; Meydani, M. Dietary Polyphenols, Inflammation, and Cancer. Nutr. Cancer 2009, 61, 807–810. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and Cancer Cell Growth. In Reviews of Physiology, Biochemistry and Pharmacology; Amara, S.G., Bamberg, E., Fleischmann, B., Gudermann, T., Hebert, S.C., Jahn, R., Lederer, W.J., Lill, R., Miyajima, A., Offermanns, S., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 79–113. ISBN 978-3-540-73800-8. [Google Scholar]
- Stoner, G.D.; Mukhtar, H. Polyphenols as Cancer Chemopreventive Agents. J. Cell. Biochem. 1995, 59, 169–180. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting Multiple Signaling Pathways by Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Assunta Dessì, M.; Spencer, J.P.E. Inhibition of P38/CREB Phosphorylation and COX-2 Expression by Olive Oil Polyphenols Underlies Their Anti-Proliferative Effects. Biochem. Biophys. Res. Commun. 2007, 362, 606–611. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Desch, S.; Schmidt, J.; Kobler, D.; Sonnabend, M.; Eitel, I.; Sareban, M.; Rahimi, K.; Schuler, G.; Thiele, H. Effect of Cocoa Products on Blood Pressure: Systematic Review and Meta-Analysis. Am. J. Hypertens. 2010, 23, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.J.; Mustonen, P.; Mattila, P.H.; Jula, A.M. Favorable Effects of Berry Consumption on Platelet Function, Blood Pressure, and HDL Cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.E.; Maalej, N.; Shanmuganayagam, D.; Folts, J.D. Grape Juice but Not Orange or Grapefruit Juice Inhibits Platelet Activity in Dogs and Monkeys (Macaca Fasciularis)1,2. J. Nutr. 1998, 128, 2307–2312. [Google Scholar] [CrossRef]
- Youdim, K.A.; Joseph, J.A. A Possible Emerging Role of Phytochemicals in Improving Age-Related Neurological Dysfunctions: A Multiplicity of Effects. Free Radic. Biol. Med. 2001, 30, 583–594. [Google Scholar] [CrossRef]
- Spencer, J.P.E. Food for Thought: The Role of Dietary Flavonoids in Enhancing Human Memory, Learning and Neuro-Cognitive Performance: Symposium on ‘Diet and Mental Health’. Proc. Nutr. Soc. 2008, 67, 238–252. [Google Scholar] [CrossRef]
- Rendeiro, C.; Spencer, J.P.E.; Vauzour, D.; Butler, L.T.; Ellis, J.A.; Williams, C.M. The Impact of Flavonoids on Spatial Memory in Rodents: From Behaviour to Underlying Hippocampal Mechanisms. Genes Nutr. 2009, 4, 251–270. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rice-Evans, C.; Williams, R.J.; Spencer, J.P.E. Activation of Pro-Survival Akt and ERK1/2 Signalling Pathways Underlie the Anti-Apoptotic Effects of Flavanones in Cortical Neurons. J. Neurochem. 2007, 103, 1355–1367. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Zaid, M.A. Insulin-Like Effect of (–)Epicatechin on Erythrocyte Membrane Acetylcholinesterase Activity in Type 2 Diabetes Mellitus. Clin. Exp. Pharmacol. Physiol. 2001, 28, 776–778. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-Hyperglycemic Effect of Diacylated Anthocyanin Derived from Ipomoea Batatas Cultivar Ayamurasaki Can Be Achieved through the α-Glucosidase Inhibitory Action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Pedret, A.; Rubió, L.; Gosalbes, M.J.; Yuste, S.; Solà, R.; Valls, R.M. Interplay between Dietary Phenolic Compound Intake and the Human Gut Microbiome in Hypertension: A Cross-Sectional Study. Food Chem. 2021, 344, 128567. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids1. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of Resveratrol on Metabolic Syndrome Components: A Systematic Review and Meta-Analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, T.; Mayama, C.; Aoki, H.; Hirayama, Y.; Shimizu, M. Antihyperglycemic Effect of Polyphenols from Acerola (Malpighia emarginata DC.) Fruit. Biosci. Biotechnol. Biochem. 2006, 70, 1813–1820. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. α-Glucosidase Inhibitory Profile of Catechins and Theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of Dietary Flavonoids with Risk of Type 2 Diabetes, and Markers of Insulin Resistance and Systemic Inflammation in Women: A Prospective Study and Cross-Sectional Analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef]
- Chen, W.-P.; Chi, T.-C.; Chuang, L.-M.; Su, M.-J. Resveratrol Enhances Insulin Secretion by Blocking KATP and KV Channels of Beta Cells. Eur. J. Pharmacol. 2007, 568, 269–277. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Mishra, N. Anti-Oxidant Effect of Quercetin on Type 2 Diabetic Erythrocytes. J. Food Biochem. 2009, 33, 404–415. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different Polyphenolic Components of Soft Fruits Inhibit α-Amylase and α-Glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Phenolic-Rich Extracts from Selected Tropical Underutilized Legumes Inhibit α-Amylase, α-Glucosidase, and Angiotensin I Converting Enzyme in Vitro. J. Basic Clin. Physiol. Pharmacol. 2012, 23, 17–25. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean Phenolic-Rich Extracts Inhibit Key-Enzymes Linked to Type 2 Diabetes (α-Amylase and α-Glucosidase) and Hypertension (Angiotensin I Converting Enzyme) in Vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Koh, L.W.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of Different Teas against Starch Digestibility by Mammalian Glycosidases. J. Agric. Food Chem. 2010, 58, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Honda, M. The Inhibition of α-Amylase by Tea Polyphenols. Agric. Biol. Chem. 1990, 54, 1939–1945. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Kim, Y.-C.; Shetty, K. Health Benefits of Traditional Corn, Beans, and Pumpkin: In Vitro Studies for Hyperglycemia and Hypertension Management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-I.; Apostolidis, E.; Shetty, K. In Vitro Studies of Eggplant (Solanum melongena) Phenolics as Inhibitors of Key Enzymes Relevant for Type 2 Diabetes and Hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin Glucosides Inhibit Glucose Uptake into Brush-Border-Membrane Vesicles of Porcine Jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary Polyphenols Decrease Glucose Uptake by Human Intestinal Caco-2 Cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef]
- Manzano, S.; Williamson, G. Polyphenols and Phenolic Acids from Strawberry and Apple Decrease Glucose Uptake and Transport by Human Intestinal Caco-2 Cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of Legumes as Part of a Low Glycemic Index Diet on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef]
- Larsen, T.M.; Dalskov, S.-M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with High or Low Protein Content and Glycemic Index for Weight-Loss Maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.-I.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef]
- Wright, A.G.; Ellis, T.P.; Ilag, L.L. Filtered Molasses Concentrate from Sugar Cane: Natural Functional Ingredient Effective in Lowering the Glycaemic Index and Insulin Response of High Carbohydrate Foods. Plant Foods Hum. Nutr. 2014, 69, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant Activity and Polyphenol Composition of Sugarcane Molasses Extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Ellis, T.P.; Wright, A.G.; Clifton, P.M.; Ilag, L.L. Postprandial Insulin and Glucose Levels Are Reduced in Healthy Subjects When a Standardised Breakfast Meal Is Supplemented with a Filtered Sugarcane Molasses Concentrate. Eur. J. Nutr. 2016, 55, 2365–2376. [Google Scholar] [CrossRef]
- Törrönen, R.; Sarkkinen, E.; Tapola, N.; Hautaniemi, E.; Kilpi, K.; Niskanen, L. Berries Modify the Postprandial Plasma Glucose Response to Sucrose in Healthy Subjects. Br. J. Nutr. 2010, 103, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Mykkänen, H.; Niskanen, L. Postprandial Glucose, Insulin, and Free Fatty Acid Responses to Sucrose Consumed with Blackcurrants and Lingonberries in Healthy Women. Am. J. Clin. Nutr. 2012, 96, 527–533. [Google Scholar] [CrossRef]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkänen, H.; Niskanen, L. Berries Reduce Postprandial Insulin Responses to Wheat and Rye Breads in Healthy Women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Possible Role for Apple Juice Phenolic Compounds in the Acute Modification of Glucose Tolerance and Gastrointestinal Hormone Secretion in Humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Payet, B.; Shum Cheong Sing, A.; Smadja, J. Comparison of the Concentrations of Phenolic Constituents in Cane Sugar Manufacturing Products with Their Antioxidant Activities. J. Agric. Food Chem. 2006, 54, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cortés, A.; Sánchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric Estimation of Total Phenolic Content and Antioxidant Capacity of Molasses and Vinasses Generated from the Sugarcane Industry. Waste Biomass Valorization 2020, 11, 3453–3463. [Google Scholar] [CrossRef]
- Kong, F.; Yu, S.; Zeng, F.; Wu, X. Phenolics Content and Inhibitory Effect of Sugarcane Molasses on α-Glucosidase and α-Amylase In Vitro. Sugar Tech 2016, 18, 333–339. [Google Scholar] [CrossRef]
- Malunga, L.N.; Joseph Thandapilly, S.; Ames, N. Cereal-Derived Phenolic Acids and Intestinal Alpha Glucosidase Activity Inhibition: Structural Activity Relationship. J. Food Biochem. 2018, 42, e12635. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Xiang, J.; Zheng, B.; Yuan, Y.; Luo, D.; Fan, J. Comparative Evaluation on Phenolic Profiles, Antioxidant Properties and α-Glucosidase Inhibitory Effects of Different Milling Fractions of Foxtail Millet. J. Cereal Sci. 2021, 99, 103217. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A Series of Cinnamic Acid Derivatives and Their Inhibitory Activity on Intestinal α-Glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional Significance and Structure–Activity Relationship of Food-Derived α-Glucosidase Inhibitors. Food Microbiol. Funct. Foods Nutr. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-Amylase by Flavonoids: Structure Activity Relationship (SAR). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.; Lin, S.; Gong, D. Inhibitory Mechanism of Apigenin on α-Glucosidase and Synergy Analysis of Flavonoids. J. Agric. Food Chem. 2016, 64, 6939–6949. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S. Flavonoids as Alpha-Glucosidase Inhibitors: Mechanistic Approaches Merged with Enzyme Kinetics and Molecular Modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Özgen, A.G.; Hamulu, F.; Bayraktar, F.; Cetínkalp, S.; Yilmaz, C.; Túzún, M.; Kabalak, T. Long-Term Treatment with Acarbose for the Treatment of Reactive Hypoglycemia. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 1998, 3, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Cież, D.; Trzewik, B.; Miszczak, K.; Tynor, G.; Bazan, B. In the Search of Glycoside-Based Molecules as Antidiabetic Agents. Top. Curr. Chem. 2019, 377, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F. Recent Advances on Natural Aryl-C-Glycoside Scaffolds: Structure, Bioactivities, and Synthesis—A Comprehensive Review. Molecules 2022, 27, 7439. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative Stress and the Use of Antioxidants in Diabetes: Linking Basic Science to Clinical Practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Weseler, A.R.; Bast, A. Oxidative Stress and Vascular Function: Implications for Pharmacologic Treatments. Curr. Hypertens. Rep. 2010, 12, 154–161. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Zhou, H.; Yan, H.; Ye, J.; Zhao, Z.; You, L.; Fu, X. Antioxidant/Antihyperglycemic Activity of Phenolics from Sugarcane (Saccharum officinarum L.) Bagasse and Identification by UHPLC-HR-TOFMS. Ind. Crops Prod. 2017, 101, 104–114. [Google Scholar] [CrossRef]
- Xu, J.; Jönsson, T.; Plaza, M.; Håkansson, Å.; Antonsson, M.; Ahrén, I.L.; Turner, C.; Spégel, P.; Granfeldt, Y. Probiotic Fruit Beverages with Different Polyphenol Profiles Attenuated Early Insulin Response. Nutr. J. 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic Index of Foods: A Physiological Basis for Carbohydrate Exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- ISO 26642:2010; International Standard-Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/obp/ui/#iso:std:iso:26642:ed-1:v1:en (accessed on 25 July 2023).
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic Index Methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
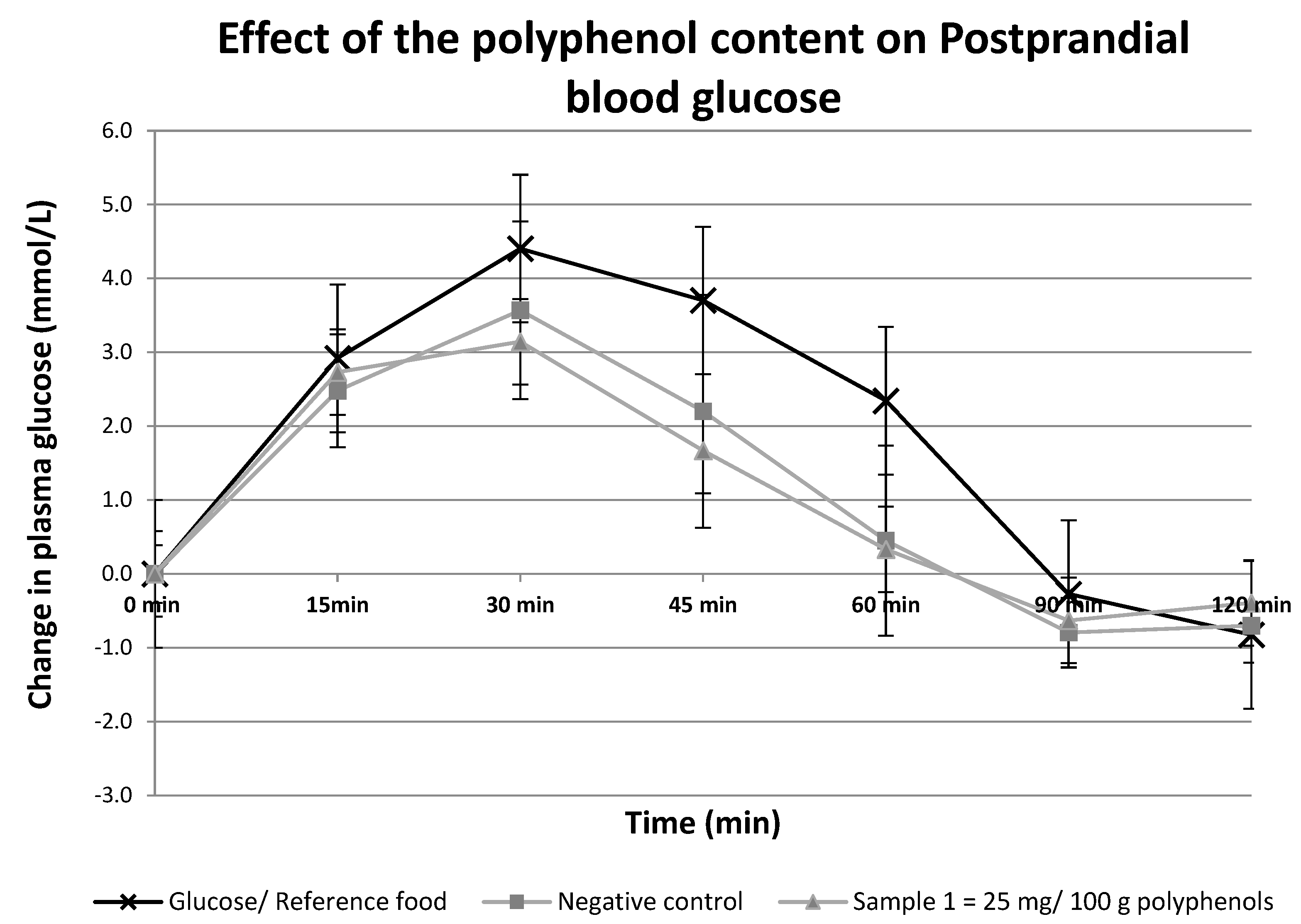

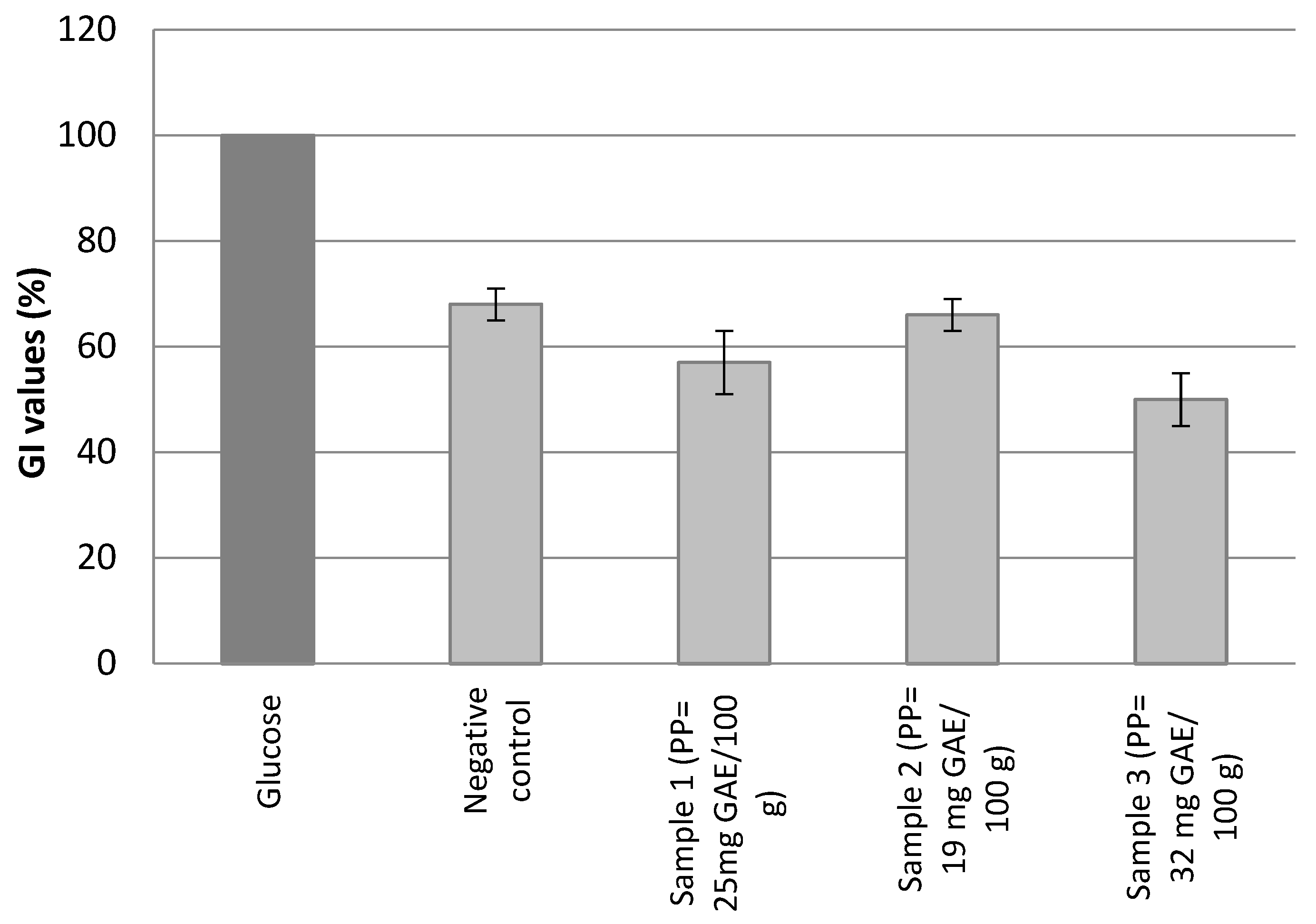
| Polyphenol Content (mg GAE/100 g) | Glycemic Index | |
|---|---|---|
| Negative control | 0 | 68.3 ± 8.2 |
| Sample 1 | 25 | 57.2 ± 5.6 |
| Sample 2 | 19 | 65.0 ± 7.2 |
| Sample 3 | 32 | 51.2 ± 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flavel, M.; Neoh, J.; Lim, K.F. Dose-Dependency of the Glycemic Response to Polyphenol-Rich Sugarcane Extract (PRSE). Biologics 2023, 3, 308-320. https://doi.org/10.3390/biologics3040016
Flavel M, Neoh J, Lim KF. Dose-Dependency of the Glycemic Response to Polyphenol-Rich Sugarcane Extract (PRSE). Biologics. 2023; 3(4):308-320. https://doi.org/10.3390/biologics3040016
Chicago/Turabian StyleFlavel, Matthew, Julian Neoh, and Kosta Fremielle Lim. 2023. "Dose-Dependency of the Glycemic Response to Polyphenol-Rich Sugarcane Extract (PRSE)" Biologics 3, no. 4: 308-320. https://doi.org/10.3390/biologics3040016





