Mechanistic Insights into Graphene Oxide Driven Photocatalysis as Co-Catalyst and Sole Catalyst in Degradation of Organic Dye Pollutants
Abstract
:1. Introduction
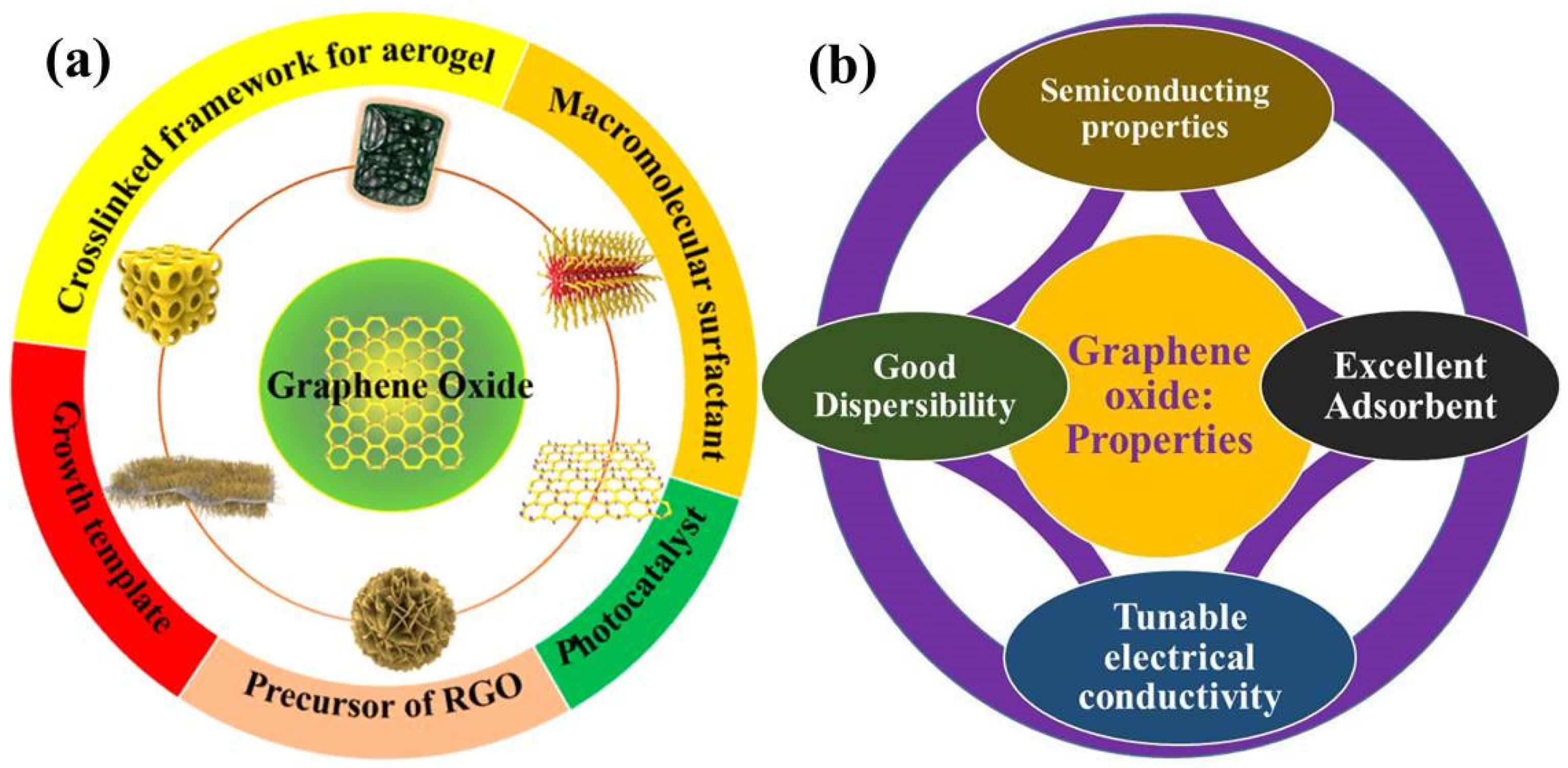
2. Graphene Oxide: Surface Chemistry/Properties Related to Photocatalysis

3. Mechanistic Insights into Graphene Oxide Driven Photocatalysis in Degradation of Organic Dye Pollutants
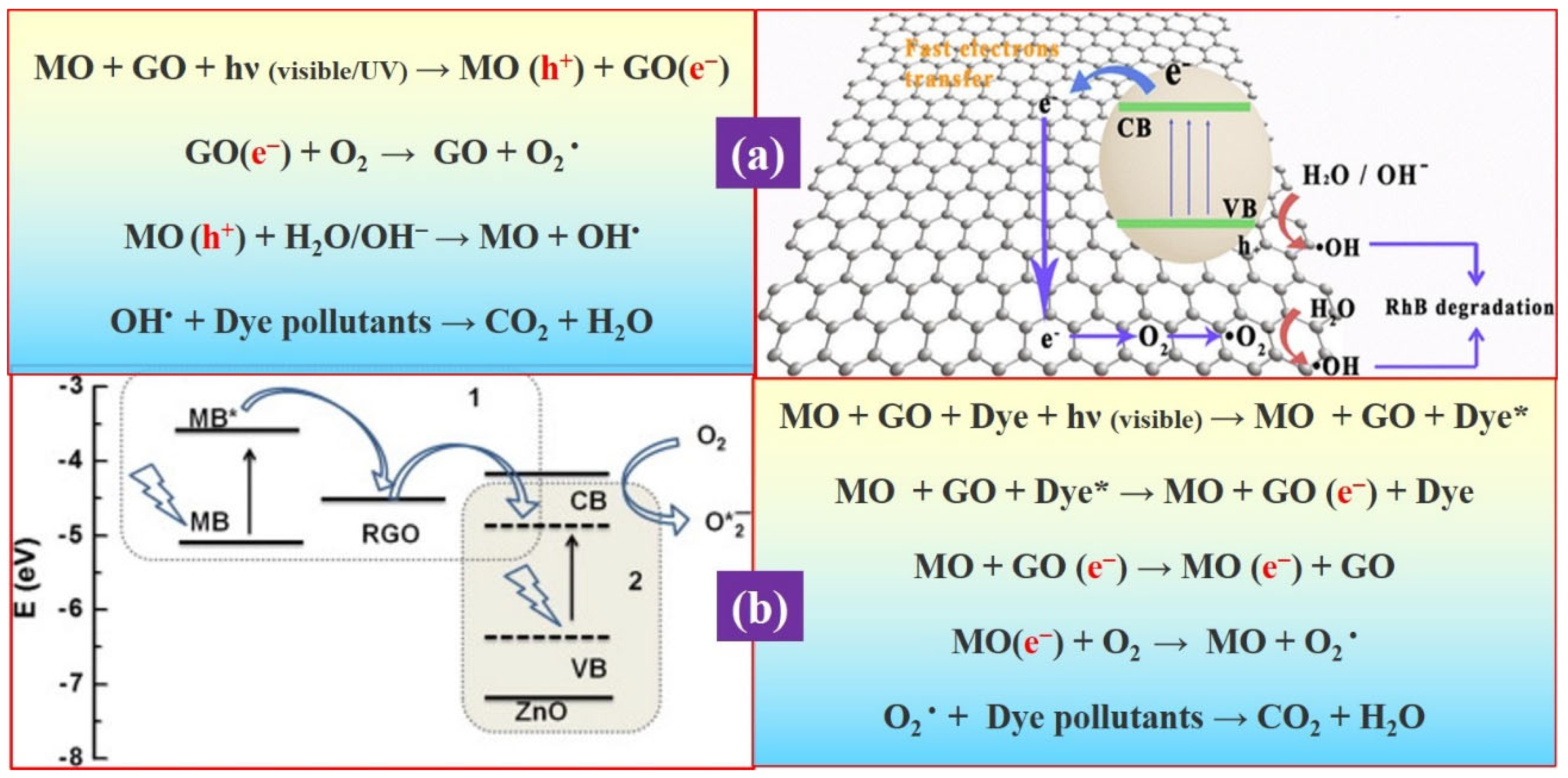
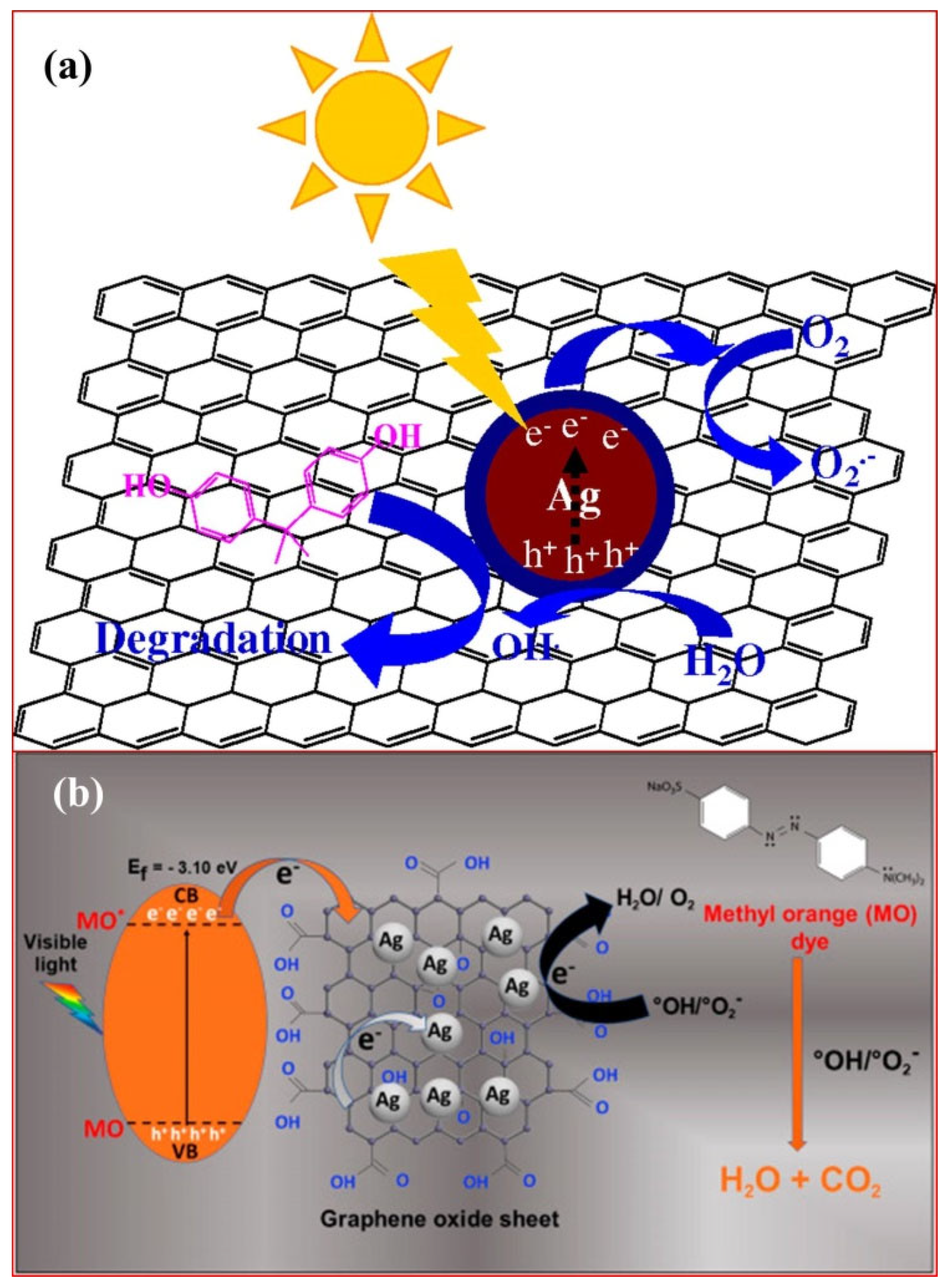
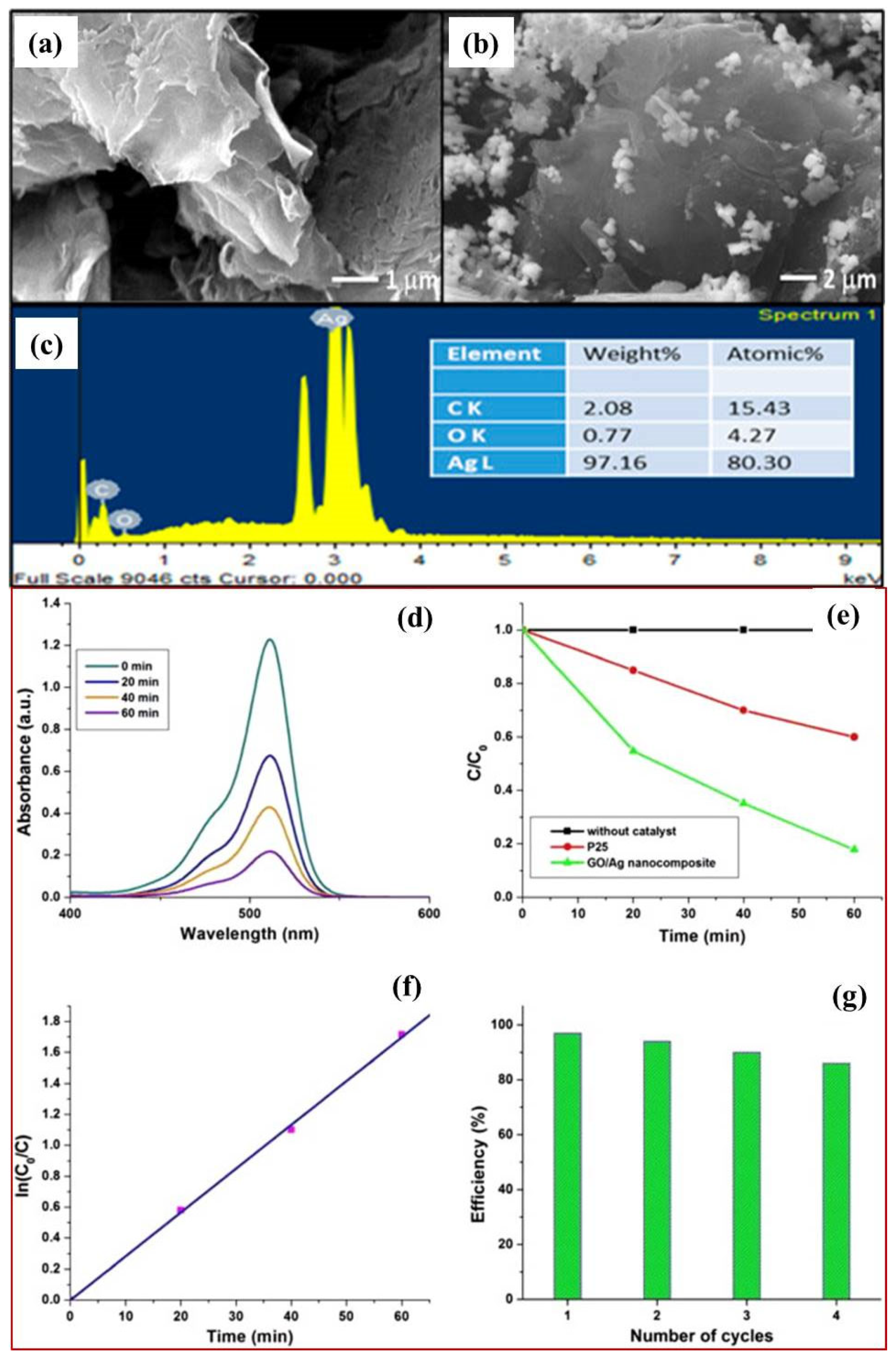
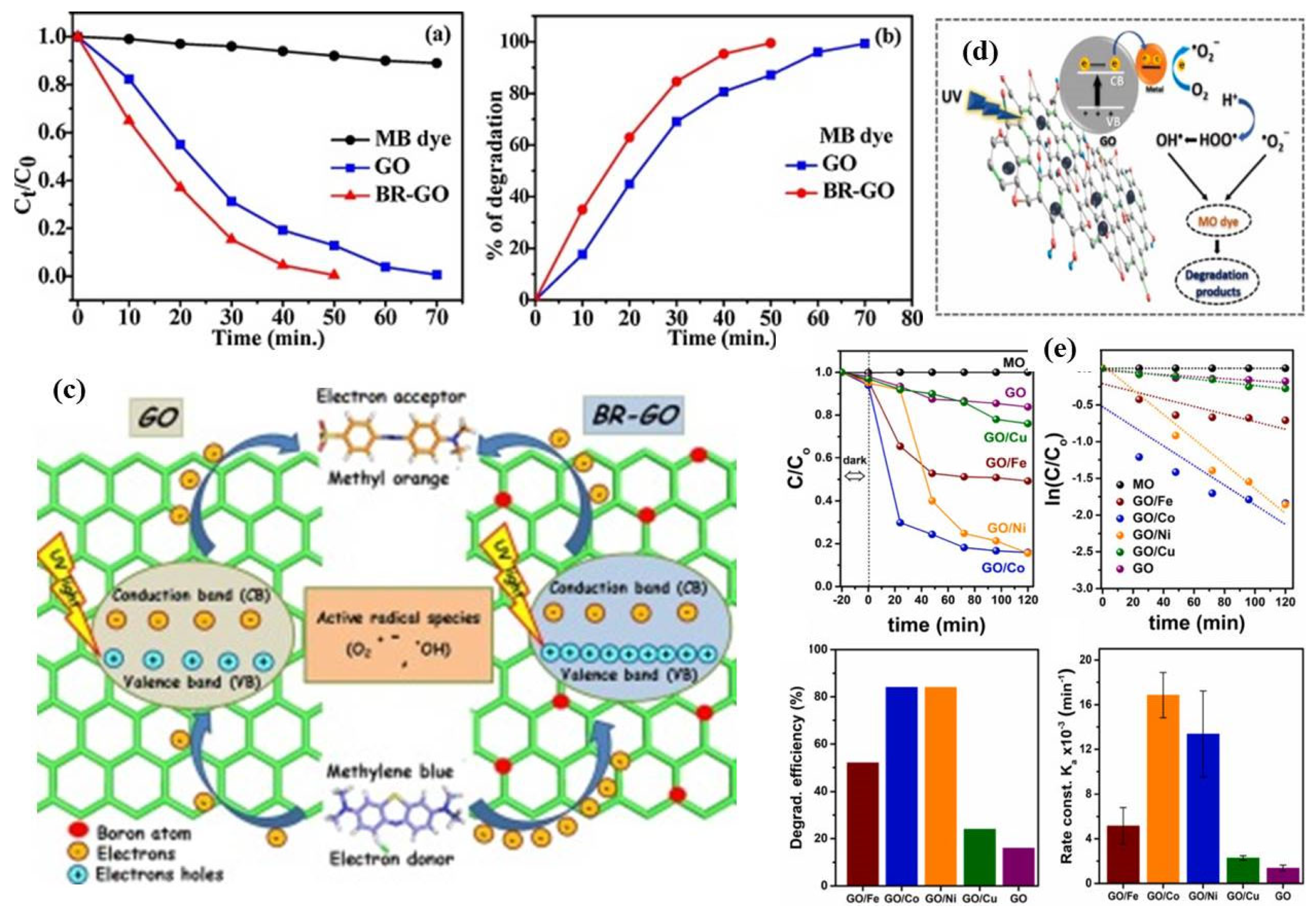
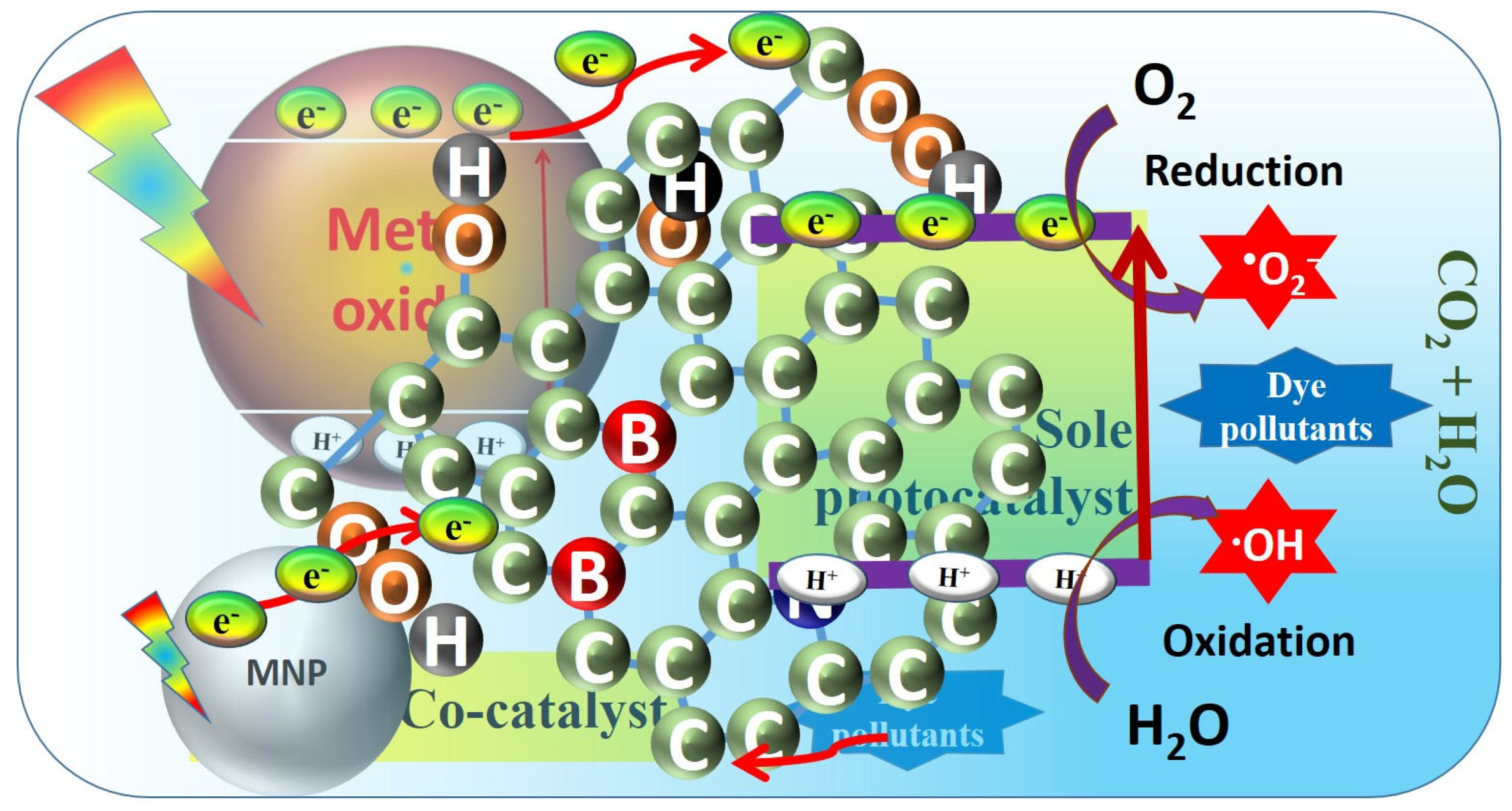
3.1. Graphene Oxide as a Co-Catalyst

3.2. As a Sole Photocatalysts
4. Summary and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Prakash, J.; Kaushal, R. An insight into the green synthesis of SiO2 nanostructures as a novel adsorbent for removal of toxic water pollutants. Environ. Res. 2022, 212, 113328. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, B.; Singh, N.; Kar, P.; Tyagi, A.; Prakash, J.; Gupta, R.K. Engineering of transition metal dichalcogenide-based 2D nanomaterials through doping for environmental applications. Mol. Syst. Des. Eng. 2019, 4, 804–827. [Google Scholar] [CrossRef]
- Prakash, J.; Harris, R.A.; Swart, H.C. Embedded plasmonic nanostructures: Synthesis, fundamental aspects and their surface enhanced Raman scattering applications. Int. Rev. Phys. Chem. 2016, 35, 353–398. [Google Scholar] [CrossRef]
- Chakraborty, A.; Samriti; Ruzimuradov, O.; Gupta, R.K.; Cho, J.; Prakash, J. TiO2 nanoflower photocatalysts: Synthesis, modifications and applications in wastewater treatment for removal of emerging organic pollutants. Environ. Res. 2022, 212, 113550. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface functionalization of bamboo leave mediated synthesized SiO2 nanoparticles: Study of adsorption mechanism, isotherms and enhanced adsorption capacity for removal of Cr (VI) from aqueous solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Chen, H.; Prakash, J.; Zheng, Y.; Sun, S. Multi-metallic catalysts for the electroreduction of carbon dioxide: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2022, 155, 111922. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, P.; Harris, R.A.; Swart, C.; Neethling, J.H.; van Vuuren, A.J.; Swart, H.C. Synthesis, characterization and multifunctional properties of plasmonic Ag–TiO2 nanocomposites. Nanotechnology 2016, 27, 355707. [Google Scholar] [CrossRef]
- Samriti; Manisha; Chen, Z.; Sun, S.; Prakash, J. Design and engineering of graphene nanostructures as independent solar-driven photocatalysts for emerging applications in the field of energy and environment. Mol. Syst. Des. Eng. 2022, 7, 213–238. [Google Scholar] [CrossRef]
- Singh, N.; Prakash, J.; Gupta, R.K. Design and engineering of high-performance photocatalytic systems based on metal oxide–graphene–noble metal nanocomposites. Mol. Syst. Des. Eng. 2017, 2, 422–439. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Singh, N.; Prakash, J.; Misra, M.; Sharma, A.; Gupta, R.K. Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds. ACS Appl. Mater. Interfaces 2017, 9, 28495–28507. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Pivin, J.C.; Swart, H.C. Noble metal nanoparticles embedding into polymeric materials: From fundamentals to applications. Adv. Colloid Interface Sci. 2015, 226, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-Q.; Li, Y.-H.; Tang, Z.-R.; Xu, Y.-J. Roles of Graphene Oxide in Heterogeneous Photocatalysis. ACS Mater. Au 2021, 1, 37–54. [Google Scholar] [CrossRef]
- Samriti; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Prakash, J. Fundamentals and applications of recyclable SERS substrates. Int. Rev. Phys. Chem. 2019, 38, 201–242. [Google Scholar] [CrossRef]
- Jamjoum, H.A.A.; Umar, K.; Adnan, R.; Razali, M.R.; Mohamad Ibrahim, M.N. Synthesis, Characterization, and Photocatalytic Activities of Graphene Oxide/metal Oxides Nanocomposites: A Review. Front. Chem. 2021, 9, 752276. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic water decontamination using graphene and ZnO coupled photocatalysts: A review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Ksari, Y.; Prakash, J.; Giovanelli, L.; Valmalette, J.-C.; Themlin, J.-M. Nitrogen-doping processes of graphene by a versatile plasma-based method. Carbon 2014, 73, 216–224. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Prakash, J.; Chen, Z.; Gauthier, M.; Sun, S. Chemical vapour deposition of graphene: Layer control, the transfer process, characterisation, and related applications. Int. Rev. Phys. Chem. 2019, 38, 149–199. [Google Scholar] [CrossRef]
- Khurshid, F.; Jeyavelan, M.; Nagarajan, S. Photocatalytic dye degradation by graphene oxide doped transition metal catalysts. Synth. Met. 2021, 278, 116832. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A. Chemically derived luminescent graphene oxide nanosheets and its sunlight driven photocatalytic activity against methylene blue dye. Opt. Mater. 2016, 62, 320–327. [Google Scholar] [CrossRef]
- Govindan, K.; Suresh, A.K.; Sakthivel, T.; Murugesan, K.; Mohan, R.; Gunasekaran, V.; Jang, A. Effect of peroxomonosulfate, peroxodisulfate and hydrogen peroxide on graphene oxide photocatalytic performances in methyl orange dye degradation. Chemosphere 2019, 237, 124479. [Google Scholar] [CrossRef]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, S.; Zhou, P.; Sun, Q.; Wang, P.; Wan, L.; Li, J.; Chen, L.; Wang, X.; Ding, S.; et al. Evolution of the band-gap and optical properties of graphene oxide with controllable reduction level. Carbon 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Abid; Sehrawat, P.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar] [CrossRef] [PubMed]
- Komba, N.; Zhang, G.; Wei, Q.; Yang, X.; Prakash, J.; Chenitz, R.; Rosei, F.; Sun, S. Iron (II) phthalocyanine/N-doped graphene: A highly efficient non-precious metal catalyst for oxygen reduction. Int. J. Hydrogen Energy 2019, 44, 18103–18114. [Google Scholar] [CrossRef]
- Farjadian, F.; Abbaspour, S.; Sadatlu, M.A.A.; Mirkiani, S.; Ghasemi, A.; Hoseini-Ghahfarokhi, M.; Mozaffari, N.; Karimi, M.; Hamblin, M.R. Recent Developments in Graphene and Graphene Oxide: Properties, Synthesis, and Modifications: A Review. ChemistrySelect 2020, 5, 10200–10219. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Mei, X.; Meng, X.; Wu, F. Hydrothermal method for the production of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostructures 2015, 68, 81–86. [Google Scholar] [CrossRef]
- Putri, L.K.; Ng, B.-J.; Ong, W.-J.; Lee, H.W.; Chang, W.S.; Chai, S.-P. Heteroatom Nitrogen- and Boron-Doping as a Facile Strategy to Improve Photocatalytic Activity of Standalone Reduced Graphene Oxide in Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Tripathi, A.; Khan, S.A.; Pivin, J.C.; Singh, F.; Tripathi, J.; Kumar, S.; Avasthi, D.K. Ion beam induced interface mixing of Ni on PTFE bilayer system studied by quadrupole mass analysis and electron spectroscopy for chemical analysis. Vacuum 2010, 84, 1275–1279. [Google Scholar] [CrossRef]
- Prakash, J.; Swart, H.C.; Zhang, G.; Sun, S. Emerging applications of atomic layer deposition for the rational design of novel nanostructures for surface-enhanced Raman scattering. J. Mater. Chem. C 2019, 7, 1447–1471. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, V.; Erasmus, L.J.B.; Duvenhage, M.M.; Sathiyan, G.; Bellucci, S.; Sun, S.; Swart, H.C. Phosphor Polymer Nanocomposite: ZnO:Tb3+ Embedded Polystyrene Nanocomposite Thin Films for Solid-State Lighting Applications. ACS Appl. Nano Mater. 2018, 1, 977–988. [Google Scholar] [CrossRef]
- Singh, J.P.; Chen, C.L.; Dong, C.L.; Prakash, J.; Kabiraj, D.; Kanjilal, D.; Pong, W.F.; Asokan, K. Role of surface and subsurface defects in MgO thin film: XANES and magnetic investigations. Superlattices Microstruct. 2015, 77, 313–324. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Prakash, J.; Zheng, Y.; Sun, S. Rational Design of Novel Catalysts with Atomic Layer Deposition for the Reduction of Carbon Dioxide. Adv. Energy Mater. 2019, 9, 1900889. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra Mathpal, M.; Prakash, J.; Viljoen, B.C.; Roos, W.D.; Swart, H.C. Band gap tailoring of cauliflower-shaped CuO nanostructures by Zn doping for antibacterial applications. J. Alloy. Compd. 2020, 832, 154968. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kumar, V.; Prakash, J.; Purohit, L.P.; Swart, H.C.; Kroon, R.E. Fabrication and characterization of nitrogen doped p-ZnO on n-Si heterojunctions. Sens. Actuators A Phys. 2016, 247, 475–481. [Google Scholar] [CrossRef]
- Prakash, J.; Singh, A.; Sathiyan, G.; Ranjan, R.; Singh, A.; Garg, A.; Gupta, R.K. Progress in tailoring perovskite based solar cells through compositional engineering: Materials properties, photovoltaic performance and critical issues. Mater. Today Energy 2018, 9, 440–486. [Google Scholar] [CrossRef]
- Gupta, T.; Samriti; Cho, J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Kumar, V.; Prakash, J.; Singh, J.P.; Chae, K.H.; Swart, C.; Ntwaeaborwa, O.M.; Swart, H.C.; Dutta, V. Role of silver doping on the defects related photoluminescence and antibacterial behaviour of zinc oxide nanoparticles. Colloids Surf. B Biointerfaces 2017, 159, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, D.; Shalini Devi, K.S.; Sathiyan, G.; Tyagi, A.; da Silva, V.A.O.P.; Janegitz, B.C.; Prakash, J.; Gupta, R.K. Novel polypyrrole-graphene oxide-gold nanocomposite for high performance hydrogen peroxide sensing application. Sens. Actuators A Phys. 2021, 328, 112769. [Google Scholar] [CrossRef]
- Ma, W.; Lv, M.; Cao, F.; Fang, Z.; Feng, Y.; Zhang, G.; Yang, Y.; Liu, H. Synthesis and characterization of ZnO-GO composites with their piezoelectric catalytic and antibacterial properties. J. Environ. Chem. Eng. 2022, 10, 107840. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Shaheen, S.; Iqbal, A.; Ikram, M.; Imran, M.; Naz, S.; Ul-Hamid, A.; Shahzadi, A.; Nabgan, W.; Haider, J.; Haider, A. Graphene oxide-ZnO nanorods for efficient dye degradation, antibacterial and in-silico analysis. Appl. Nanosci. 2022, 12, 165–177. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Role of oxygen functionalities on the synthesis of photocatalytically active graphene–TiO2 composites. Appl. Catal. B Environ. 2014, 158–159, 329–340. [Google Scholar] [CrossRef]
- Víctor-Román, S.; García-Bordejé, E.; Hernández-Ferrer, J.; González-Domínguez, J.M.; Ansón-Casaos, A.; Silva, A.M.T.; Maser, W.K.; Benito, A.M. Controlling the surface chemistry of graphene oxide: Key towards efficient ZnO-GO photocatalysts. Catal. Today 2020, 357, 350–360. [Google Scholar] [CrossRef]
- Lin, Y.; Hong, R.; Chen, H.; Zhang, D.; Xu, J. Green Synthesis of ZnO-GO Composites for the Photocatalytic Degradation of Methylene Blue. J. Nanomater. 2020, 2020, 4147357. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Pham, V.H.; Shin, E.W.; Pham, H.-D.; Kim, S.; Chung, J.S.; Kim, E.J.; Hur, S.H. The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chem. Eng. J. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Micochova, P.; Chadha, A.; Hesseloj, T.; Fraternali, F.; Ramsden, J.; Gupta, R. Rapid inactivation of SARS-CoV-2 by titanium dioxide surface coating [version 2; peer review: 2 approved]. Wellcome Open Res. 2021, 6, 56. [Google Scholar] [CrossRef]
- Iqbal, W.; Tian, B.; Anpo, M.; Zhang, J. Single-step solvothermal synthesis of mesoporous anatase TiO2-reduced graphene oxide nanocomposites for the abatement of organic pollutants. Res. Chem. Intermed. 2017, 43, 5187–5201. [Google Scholar] [CrossRef]
- Tien, H.N.; Luan, V.H.; Hoa, L.T.; Khoa, N.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Hur, S.H. One-pot synthesis of a reduced graphene oxide–zinc oxide sphere composite and its use as a visible light photocatalyst. Chem. Eng. J. 2013, 229, 126–133. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Allabadi, O.; Aljarrah, M.T. Photocatalytic Activity of Graphene Oxide/Zinc Oxide Nanocomposites with Embedded Metal Nanoparticles for the Degradation of Organic Dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef] [PubMed]
- Oppong, S.O.-B.; Anku, W.W.; Shukla, S.K.; Agorku, E.S.; Govender, P.P. Photocatalytic degradation of indigo carmine using Nd-doped TiO2-decorated graphene oxide nanocomposites. J. Sol-Gel Sci. Technol. 2016, 80, 38–49. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra Mathpal, M.; Jagannath, G.; Prakash, J.; Maze, J.-R.; Roos, W.D.; Swart, H.C. Optical limiting applications of resonating plasmonic Au nanoparticles in a dielectric glass medium. Nanotechnology 2021, 32, 345709. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, G.; Prakash, J.; Sun, S. 3D Graphene and Its Nanocomposites: From Synthesis to Multifunctional Applications. In Graphene Functionalization Strategies: From Synthesis to Applications; Khan, A., Jawaid, M., Neppolian, B., Asiri, A.M., Eds.; Springer: Singapore, 2019; pp. 363–388. [Google Scholar]
- Verma, S.; Mal, D.S.; de Oliveira, P.R.; Janegitz, B.C.; Prakash, J.; Gupta, R.K. A facile synthesis of novel polyaniline/graphene nanocomposite thin films for enzyme-free electrochemical sensing of hydrogen peroxide. Mol. Syst. Des. Eng. 2022, 7, 158–170. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, V.; Kroon, R.E.; Asokan, K.; Rigato, V.; Chae, K.H.; Gautam, S.; Swart, H.C. Optical and surface enhanced Raman scattering properties of Au nanoparticles embedded in and located on a carbonaceous matrix. Phys. Chem. Chem. Phys. 2016, 18, 2468–2480. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra Mathpal, M.; Prakash, J.; Jagannath, G.; Roos, W.D.; Swart, H.C. Plasmonic and nonlinear optical behavior of nanostructures in glass matrix for photonics application. Mater. Res. Bull. 2020, 125, 110799. [Google Scholar] [CrossRef]
- Prakash, J.; Tripathi, A.; Gautam, S.; Chae, K.H.; Song, J.; Rigato, V.; Tripathi, J.; Asokan, K. Phenomenological understanding of dewetting and embedding of noble metal nanoparticles in thin films induced by ion irradiation. Mater. Chem. Phys. 2014, 147, 920–924. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Prakash, J.; Hamad, S.; Rao, S.V.; Viljoen, B.C.; Duvenhage, M.-M.; Njoroge, E.G.; Roos, W.D.; Swart, H.C. Study of Tunable Plasmonic, Photoluminscence, and Nonlinear Optical Behavior of Ag Nanoclusters Embedded in a Glass Matrix for Multifunctional Applications. Phys. Status Solidi 2019, 216, 1800768. [Google Scholar] [CrossRef]
- Manchala, S.; Nagappagari, L.R.; Venkatakrishnan, S.M.; Shanker, V. Solar-Light Harvesting Bimetallic Ag/Au Decorated Graphene Plasmonic System with Efficient Photoelectrochemical Performance for the Enhanced Water Reduction Process. ACS Appl. Nano Mater. 2019, 2, 4782–4792. [Google Scholar] [CrossRef]
- Fan, B.; Guo, H.; Shi, J.; Shi, C.; Jia, Y.; Wang, H.; Chen, D.; Yang, Y.; Lu, H.; Xu, H.; et al. Facile One-Pot Preparation of Silver/Reduced Graphene Oxide Nanocomposite for Cancer Photodynamic and Photothermal Therapy. J. Nanosci. Nanotechnol. 2016, 16, 7049–7054. [Google Scholar] [CrossRef]
- Pusty, M.; Rana, A.K.; Kumar, Y.; Sathe, V.; Sen, S.; Shirage, P. Synthesis of Partially Reduced Graphene Oxide/Silver Nanocomposite and Its Inhibitive Action on Pathogenic Fungi Grown Under Ambient Conditions. ChemistrySelect 2016, 1, 4235–4245. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Jana, N.R. Reduced Graphene Oxide-Silver Nanoparticle Composite as Visible Light Photocatalyst for Degradation of Colorless Endocrine Disruptors. ACS Appl. Mater. Interfaces 2014, 6, 20085–20092. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Raza, A.; Imran, M.; Ul-Hamid, A.; Shahbaz, A.; Ali, S. Hydrothermal Synthesis of Silver Decorated Reduced Graphene Oxide (rGO) Nanoflakes with Effective Photocatalytic Activity for Wastewater Treatment. Nanoscale Res. Lett. 2020, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, J.; Liu, G.; Xu, L.; Tian, Y.; Jiao, A.; Chen, M. Graphene oxide-grafted plasmonic Au@Ag nanoalloys with improved synergistic effects for promoting hot carrier-driven photocatalysis under visible light irradiation. Nanotechnology 2020, 32, 125401. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, F.; Liu, T.; Zhang, N.; Wang, Y. Design of novel structured Au/g-C3N4 nanosheet/reduced graphene oxide nanocomposites for enhanced visible light photocatalytic activities. Sustain. Energy Fuels 2020, 4, 4086–4095. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Abd El-Baki, R.F.; Alsaaq, F.; Orzechowska, S.; Hamad, D. Composite Nanoarchitectonics of Graphene Oxide for Better Understanding on Structural Effects on Photocatalytic Performance for Methylene Blue Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1191–1205. [Google Scholar] [CrossRef]
- Mangalam, J.; Kumar, M.; Sharma, M.; Joshi, M. High adsorptivity and visible light assisted photocatalytic activity of silver/reduced graphene oxide (Ag/rGO) nanocomposite for wastewater treatment. Nano-Struct. Nano-Objects 2019, 17, 58–66. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kim, B.-K.; Jo, Y.-L.; Shim, J.-J. Ag@graphene oxide nanocomposite as an efficient visible-light plasmonic photocatalyst for the degradation of organic pollutants: A facile green synthetic approach. Mater. Chem. Phys. 2014, 143, 1452–1461. [Google Scholar] [CrossRef]
- Kumar, A.; Sadanandhan, A.M.; Jain, S.L. Silver doped reduced graphene oxide as a promising plasmonic photocatalyst for oxidative coupling of benzylamines under visible light irradiation. New J. Chem. 2019, 43, 9116–9122. [Google Scholar] [CrossRef]
- Zhang, L.; Du, L.; Yu, X.; Tan, S.; Cai, X.; Yang, P.; Gu, Y.; Mai, W. Significantly Enhanced Photocatalytic Activities and Charge Separation Mechanism of Pd-Decorated ZnO–Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Basak, D. One-step nano-engineering of dispersed Ag–ZnO nanoparticles’ hybrid in reduced graphene oxide matrix and its superior photocatalytic property. CrystEngComm 2013, 15, 7606–7614. [Google Scholar] [CrossRef]
- Gea, S.; Situmorang, S.A.; Pasaribu, N.; Piliang, A.F.R.; Attaurrazaq, B.; Sari, R.M.; Pasaribu, K.M.; Goutianos, S. Facile synthesis of ZnO–Ag nanocomposite supported by graphene oxide with stabilised band-gap and wider visible-light region for photocatalyst application. J. Mater. Res. Technol. 2022, 19, 2730–2741. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Li, F.; Yang, Y.; Pan, Y.; Peng, Y.; Yang, X.; Zeng, G. A novel photocatalytic membrane decorated with RGO-Ag-TiO2 for dye degradation and oil–water emulsion separation. J. Chem. Technol. Biotechnol. 2018, 93, 761–775. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Liu, R.; Wang, X.; Zhu, Y.; Zhang, J. SERS and the photo-catalytic performance of Ag/TiO2/graphene composites. Opt. Mater. Express 2018, 8, 704–717. [Google Scholar] [CrossRef]
- Qi, H.-P.; Wang, H.-L.; Zhao, D.-Y.; Jiang, W.-F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yu, H.; Zuo, Y.; Gao, J.; He, G.; Sun, Z. Facile fabrication of Ag/graphene oxide/TiO2 nanorod array as a powerful substrate for photocatalytic degradation and surface-enhanced Raman scattering detection. Appl. Catal. B Environ. 2019, 252, 174–186. [Google Scholar] [CrossRef]
- Leong, K.H.; Sim, L.C.; Bahnemann, D.; Jang, M.; Ibrahim, S.; Saravanan, P. Reduced graphene oxide and Ag wrapped TiO2 photocatalyst for enhanced visible light photocatalysis. APL Mater. 2015, 3, 104503. [Google Scholar] [CrossRef]
- Kisielewska, A.; Spilarewicz-Stanek, K.; Cichomski, M.; Kozłowski, W.; Piwoński, I. The role of graphene oxide and its reduced form in the in situ photocatalytic growth of silver nanoparticles on graphene-TiO2 nanocomposites. Appl. Surf. Sci. 2022, 576, 151759. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Mohan, R.; Kim, S.-J. Graphene oxide as a photocatalytic material. Appl. Phys. Lett. 2011, 98, 244101. [Google Scholar] [CrossRef]
- Siong, V.L.E.; Tai, X.H.; Lee, K.M.; Juan, J.C.; Lai, C.W. Unveiling the enhanced photoelectrochemical and photocatalytic properties of reduced graphene oxide for photodegradation of methylene blue dye. RSC Adv. 2020, 10, 37905–37915. [Google Scholar] [CrossRef]
- Siong, V.L.E.; Lee, K.M.; Juan, J.C.; Lai, C.W.; Tai, X.H.; Khe, C.S. Removal of methylene blue dye by solvothermally reduced graphene oxide: A metal-free adsorption and photodegradation method. RSC Adv. 2019, 9, 37686–37695. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Koinuma, M.; Ida, S.; Hayami, S.; Taniguchi, T.; Hatakeyama, K.; Tateishi, H.; Watanabe, Y.; Amano, S. Photoreaction of Graphene Oxide Nanosheets in Water. J. Phys. Chem. C 2011, 115, 19280–19286. [Google Scholar] [CrossRef]
- Singh, M.; Bajaj, N.K.; Bhardwaj, A.; Singh, P.; Kumar, P.; Sharma, J. Study of photocatalytic and antibacterial activities of graphene oxide nanosheets. Adv. Compos. Hybrid Mater. 2018, 1, 759–765. [Google Scholar] [CrossRef]
- Wong, C.P.P.; Lai, C.W.; Lee, K.M.; Hamid, S.B.A. Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5. Materials 2015, 8, 7118–7128. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B Environ. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Xue, B.; Zou, Y. High photocatalytic activity of ZnO–graphene composite. J. Colloid Interface Sci. 2018, 529, 306–313. [Google Scholar] [CrossRef]
- Singh, M.; Kaushal, S.; Singh, P.; Sharma, J. Boron doped graphene oxide with enhanced photocatalytic activity for organic pollutants. J. Photochem. Photobiol. A Chem. 2018, 364, 130–139. [Google Scholar] [CrossRef]
- Junaid, M.; Khir, M.H.M.; Witjaksono, G.; Tansu, N.; Saheed, M.S.M.; Kumar, P.; Ullah, Z.; Yar, A.; Usman, F. Boron-Doped Reduced Graphene Oxide with Tunable Bandgap and Enhanced Surface Plasmon Resonance. Molecules 2020, 25, 3646. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Zhang, Y.; Zhang, N.; Xu, Y.-J. New insight into the enhanced visible light photocatalytic activity over boron-doped reduced graphene oxide. Nanoscale 2015, 7, 7030–7034. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.H.; Lai, C.W.; Yang, T.C.K.; Chen, C.-Y.; Abdullah, A.H.; Lee, K.M.; Juan, J.C. Effective oxygenated boron groups of boron-doped photoreduced graphene oxide for photocatalytic removal of volatile organic compounds. J. Environ. Chem. Eng. 2022, 10, 108047. [Google Scholar] [CrossRef]



| S. No | GO Based Photocatalysts | Role of GO | Uv/Visible Radiation/Other Conditions | Organic Dye Pollutants | Results/Photocatalytic Efficiency/Rate | Ref. |
|---|---|---|---|---|---|---|
| 1. | Co and Ni-modified GO | Sole photocatalyst | UV light | MO | Metal doping improved the properties resulting in high photocurrent, photodegradation efficiency ~84%. Rate constants = 13 × 10−3 min−1 and 16 × 10−3 min−1). | [21] |
| 2. | GO nanosheets | Sole photocatalyst | Visible light | MB | Photodegradation efficiency 60%. | [22] |
| 3. | Single layer GO modified by electron scavenger | Sole photocatalyst | UV light | MO | Band gap = 3.19–4.4 eV, photocatalytic efficiency was 24% and enhanced 3 times greater in presence of electron scavenger | [23] |
| 4. | ZnO-GO nanocomposites | Co-catalyst | Under the darkness ultrasound-driven piezoelectric catalysis effect, visible light | MB, RhB and MO | ZnO-GO nanocomposites exhibit stronger piezoelectric catalytic activity compared with the pure ZnO, formation of ROS | [43] |
| 5. | GO-ZnO nanorods | Co-catalyst | UV light | MB | Excellent photoactivity due to strong interface coupling between ZnO and Go | [45] |
| 6. | GO–TiO2 nanocomposite | Co-catalyst | Near-UV/Vis and visible light. | DP | Higher photocatalytic degradation efficiency as compared to bare TiO2 and rate of 83.9 × 10−3 min−1 | [46] |
| 7. | GO-ZnO nanocomposite | Co-catalyst | UV light | MB | Photodegradation efficiency ~80% in 70 min, interface interactions, photo-induced charge transfer interactions, high performance and recyclability | [47] |
| 8. | ZnO-GO nanocomposite | Co-catalyst | UV light | MB | Photodegradation efficiency 97.6% in 90 min and the first-order reaction rate 0.04401 min−1. | [48] |
| 9. | GO–TiO2 nanocomposite | Adsorbent, electron acceptor and photosensitizer | UV and visible light | MB | Photodegradation efficiency of 90 and 95% with rate of 72.25 × 10−3 and 23.66 × 10−3 min−1 for under UV and visible light respectively | [49] |
| 10. | TiO2–rGO nanocomposites | Co-catalyst | Visible light | RhB, phenol | Photodegradation efficiency 85% for RhB in 90 min, 100% degradation of phenol in 150 min, strong interfacial contact and charge separation. | [51] |
| 11. | GO–ZnO–Cu/Ag nanocomposite | Co-catalyst | Sunlight | MB | Catalytic activity of 84% (Cu) 100% after 40 min (Ag) rate constant of 0.1112 min–1 | [53] |
| 12. | GO and Ag@rGO nanocomposite | Both | Visible light | MB | % degradation up to 100% in 120 min. rate 0.1300 min−1 (GO) to 0.7459 min−1 (Ag@rGO) | [67] |
| 13. | Au@Ag/GO nanocomposite | Co-catalyst | Visible light | tetracycline hydrochloride | 99.36% photodegradation in 70 min. | [68] |
| 14. | Au/g-C3N4/rGO | Co-catalyst | Visible light | MB | Photodegradation rate 6 times higher than pure g-C3N4 | [69] |
| 15. | porous GO, Au–RGO, and GO-Au-ZnO nanocomposite | Porous GO as sole catalysts | UV-visible light | MB | Highest Photodegradation efficiency 97% for porous GO with rate constant 20.0 × 10–3 min–1 | [70] |
| 16. | GO and Ag/rGO | Co-catalyst | Visible light | MO | Photodegradation rate for Ag/rGO was 0.048 min−1 and, for GO it was 0.02 min−1 | [71] |
| 17. | ZnO–Ag-GO | Co-catalyst | Visible light | - | Band gap of 2.75 eV | [76] |
| 18. | Ag-modified GO-TiO2 | Co-catalyst | Sunlight | RhB | 100% RhB removal in 180 min with rate constant of 4.65 × 10–3 min–1 | [79] |
| 19. | rGO nanosheet | Sole catalyst | UV-light | MB | Band gap = 3.10 eV, the pseudo-first order rate constant of rGO was 0.070 h−1 which was remarkably 2.5 times higher than the pristine GO with rate 0.028 h−1. | [84] |
| 20. | rGO nanosheet | Sole catalyst | UV-light | MB | Photocatalytic degradation efficiency of 98.57% with rate 0.711 h−1 | [85] |
| 21. | GO nanosheet | Sole catalyst | UV-light | Congo red (CR) | Photodegradatin efficiency more than 90% in 120 min with rate constant of 0.0359 min−1 | [87] |
| 22. | B doped GO | Sole catalyst | UV-light | MB and MO | Band gap of GO and B doped GO was 2.8 eV and 3.00 eV respectively. MB dye degradation 100% in 50 min by doped GO while 70% by GO. MO degradation 100% in 100 min by doped GO while 50% only by GO. | [91] |
| 23. | B doped GO | Sole catalyst | UV-light | VoCs | Remove 80% of the VoCs within 6 h (0.283 h−1) | [94] |
| 24. | B doped rGO | Sole catalyst | Visible light | RhB | B doped rGO showed significantly higher photocatalytic activity than non-doped RGO | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, J. Mechanistic Insights into Graphene Oxide Driven Photocatalysis as Co-Catalyst and Sole Catalyst in Degradation of Organic Dye Pollutants. Photochem 2022, 2, 651-671. https://doi.org/10.3390/photochem2030043
Prakash J. Mechanistic Insights into Graphene Oxide Driven Photocatalysis as Co-Catalyst and Sole Catalyst in Degradation of Organic Dye Pollutants. Photochem. 2022; 2(3):651-671. https://doi.org/10.3390/photochem2030043
Chicago/Turabian StylePrakash, Jai. 2022. "Mechanistic Insights into Graphene Oxide Driven Photocatalysis as Co-Catalyst and Sole Catalyst in Degradation of Organic Dye Pollutants" Photochem 2, no. 3: 651-671. https://doi.org/10.3390/photochem2030043






