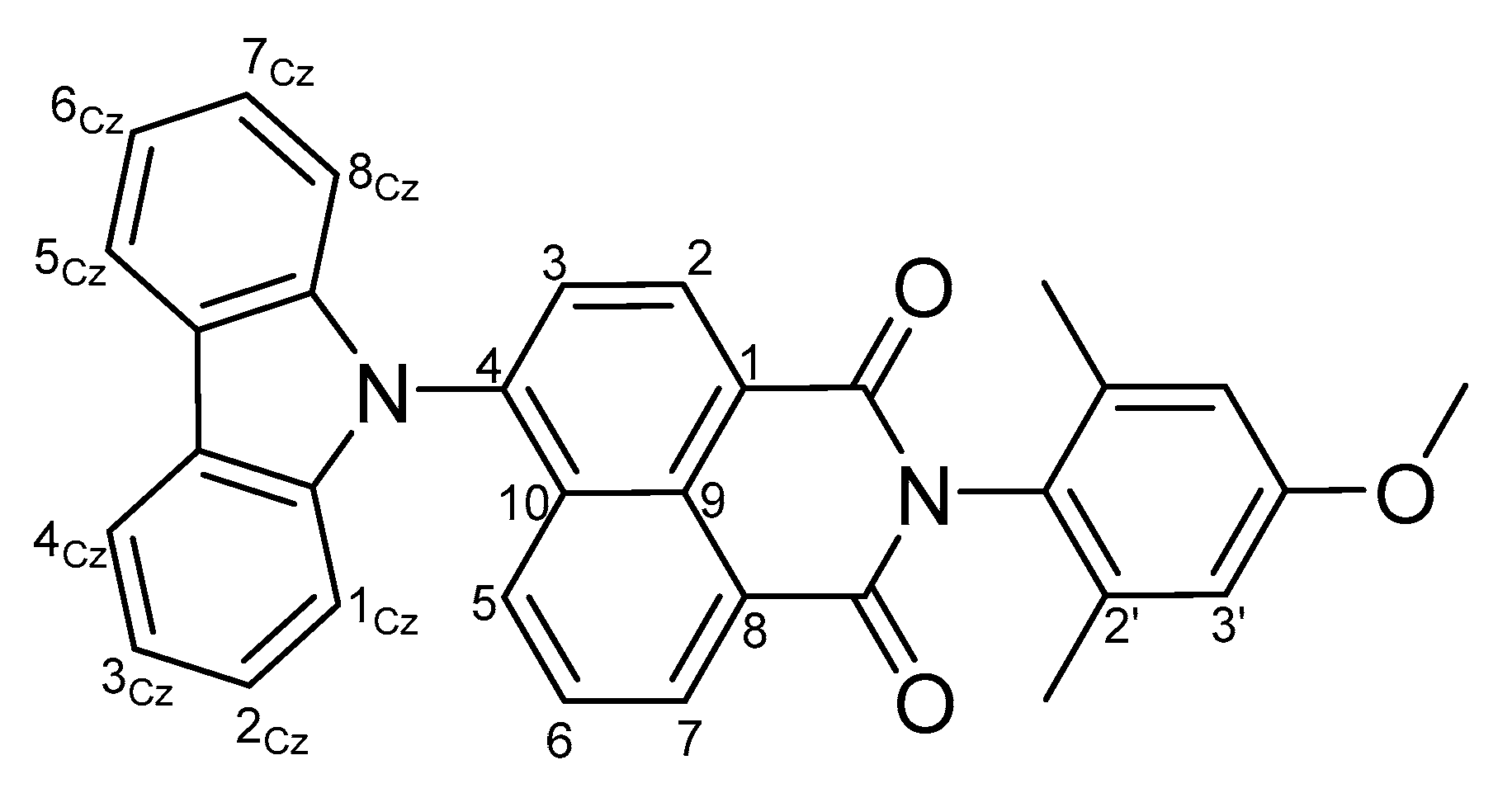
Probing the Local Polarity in Biocompatible Nanocarriers with Solvatofluorochromism of a 4-Carbazole-1,8-naphthalimide Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. NMR Spectroscopy
2.2.2. Mass Spectrometry
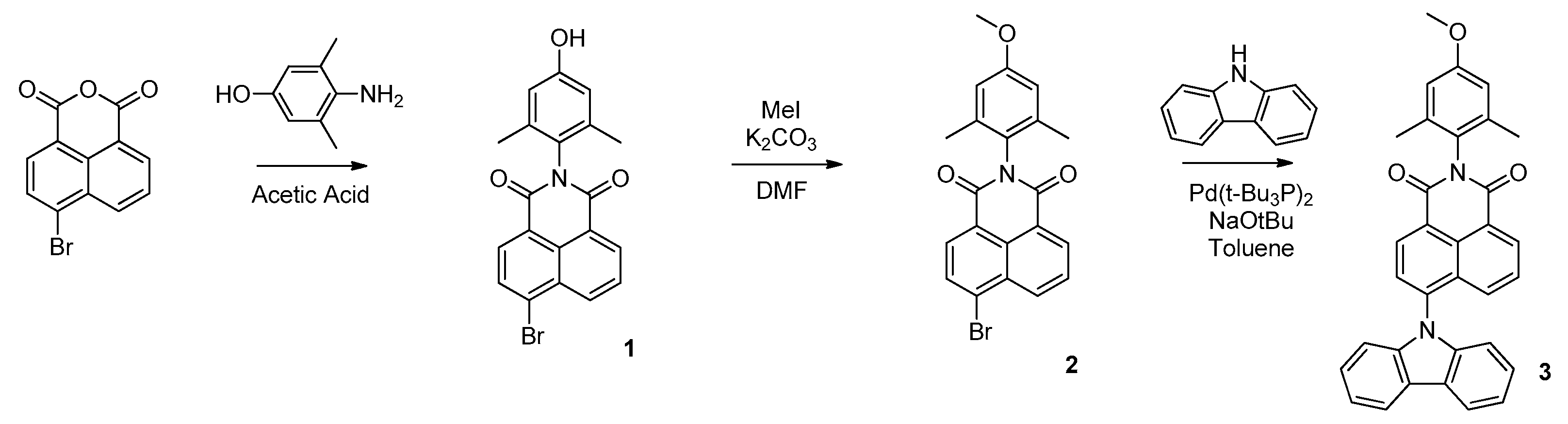
2.2.3. Synthesis
Synthesis of 4-Bromo-N-(4-hydroxy-2,6-dimethylphenyl)-1,8-naphthalimide (1)
Synthesis of 4-Bromo-N-(4-methoxy-2,6-dimethylphenyl)-1,8-naphthalimide (2)
Synthesis of 4-Carbazole-N-(4-methoxy-2,6-dimethylphenyl)-1,8-naphthalimide (3)
2.2.4. Nanoparticles Preparation
2.2.5. Dye-Loading of Nanocarriers
2.2.6. Optical Spectroscopy
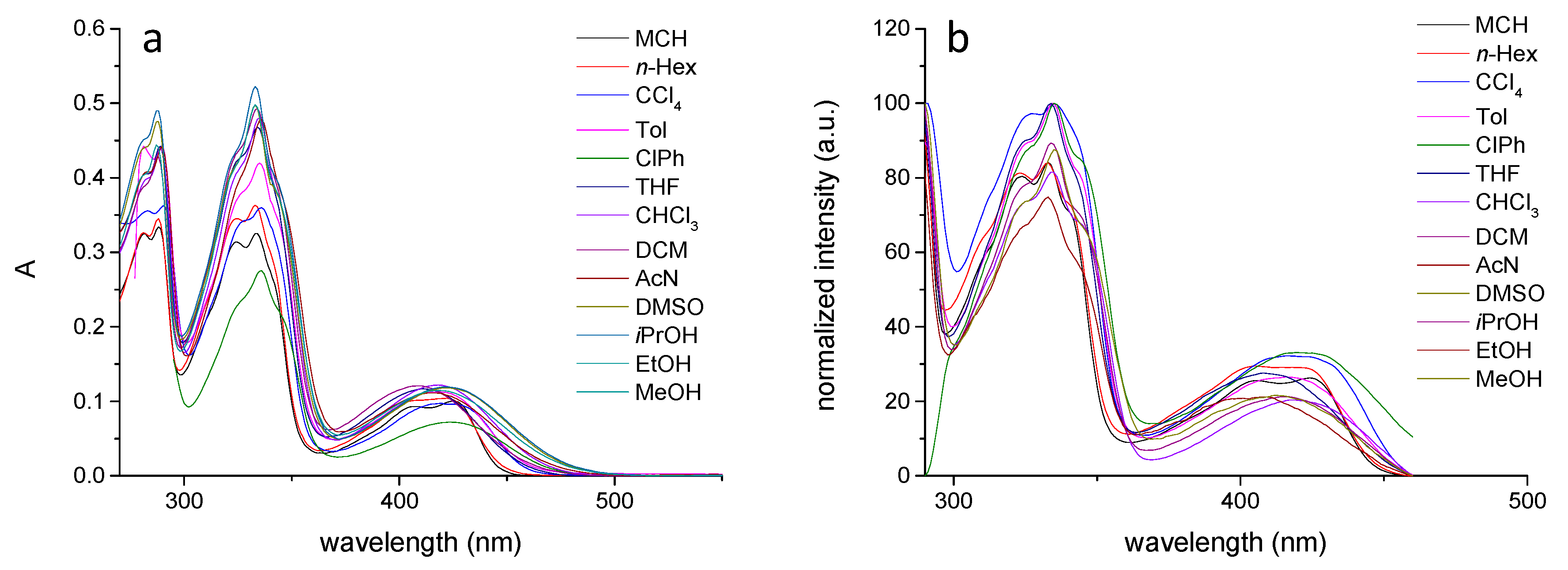
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Solvents | ET(30) a (kcal mol−1) | n | ε | f(η2, εS) b |
|---|---|---|---|---|
| MCH | 32.2 | 1.423 | 2.02 | −0.000580992 |
| n-Hexane | 31.1 | 1.387 | 1.92 | −0.000481927 |
| CCl4 | 32.4 | 1.4601 | 2.24 | 0.011243472 |
| Toluene | 33.9 | 1.4968 | 2.38 | 0.013264985 |
| ClPh | 36.8 | 1.5248 | 5.62 | 0.142936293 |
| THF | 37.4 | 1.407 | 7.58 | 0.209640381 |
| DCM | 40.7 | 1.4125 | 8.93 | 0.221048974 |
| AcN | 45.6 | 1.344 | 35.94 | 0.304609347 |
| DMSO | 45.1 | 1.479 | 46.68 | 0.263179328 |
| iPrOH | 19.92 | 1.3776 | 19.92 | 0.276060403 |
| EtOH | 24.55 | 1.3617 | 24.55 | 0.288635801 |
| MeOH | 32.7 | 1.3292 | 32.7 | 0.308276522 |
| Solvents | υa (nm) | υa (cm−1) | υf (nm) | υf (cm−1) | Δυ (cm−1) |
|---|---|---|---|---|---|
| MCH | 475 | 21,053 | 528 | 18,939 | 2113 |
| n-Hexane | 490 | 20,408 | 543 | 18,416 | 1992 |
| Toluene | 494 | 20,243 | 569 | 17,575 | 2668 |
| ClPh | 522 | 19,157 | 597 | 16,750 | 2407 |
| THF | 508 | 19,685 | 594 | 16,835 | 2850 |
| DCM | 521 | 19,194 | 601 | 16,639 | 2555 |
| AcN | 516 | 19,380 | 614 | 16,287 | 3093 |
| DMSO | 534 | 18,727 | 630 | 15,873 | 2854 |
| iPrOH | 525 | 19,048 | 624 | 16,026 | 3022 |
| EtOH | 532 | 18,797 | 631 | 15,848 | 2949 |
| MeOH | 534 | 18,727 | 636 | 15,723 | 3003 |
| Solvents | υa (nm) | υa (cm−1) | υf (nm) | υf (cm−1) | Δυ (cm−1) |
|---|---|---|---|---|---|
| MCH | 475 | 21,053 | 528 | 18,939 | 2113 |
| n-Hexane | 490 | 20,408 | 543 | 18,416 | 1992 |
| Toluene | 494 | 20,243 | 569 | 17,575 | 2668 |
| ClPh | 522 | 19,157 | 597 | 16,750 | 2407 |
| THF | 508 | 19,685 | 594 | 16,835 | 2850 |
| DCM | 521 | 19,194 | 601 | 16,639 | 2555 |
| AcN | 516 | 19,380 | 614 | 16,287 | 3093 |
| DMSO | 534 | 18,727 | 630 | 15,873 | 2854 |
| iPrOH | 525 | 19,048 | 624 | 16,026 | 3022 |
| EtOH | 532 | 18,797 | 631 | 15,848 | 2949 |
| MeOH | 534 | 18,727 | 636 | 15,723 | 3003 |
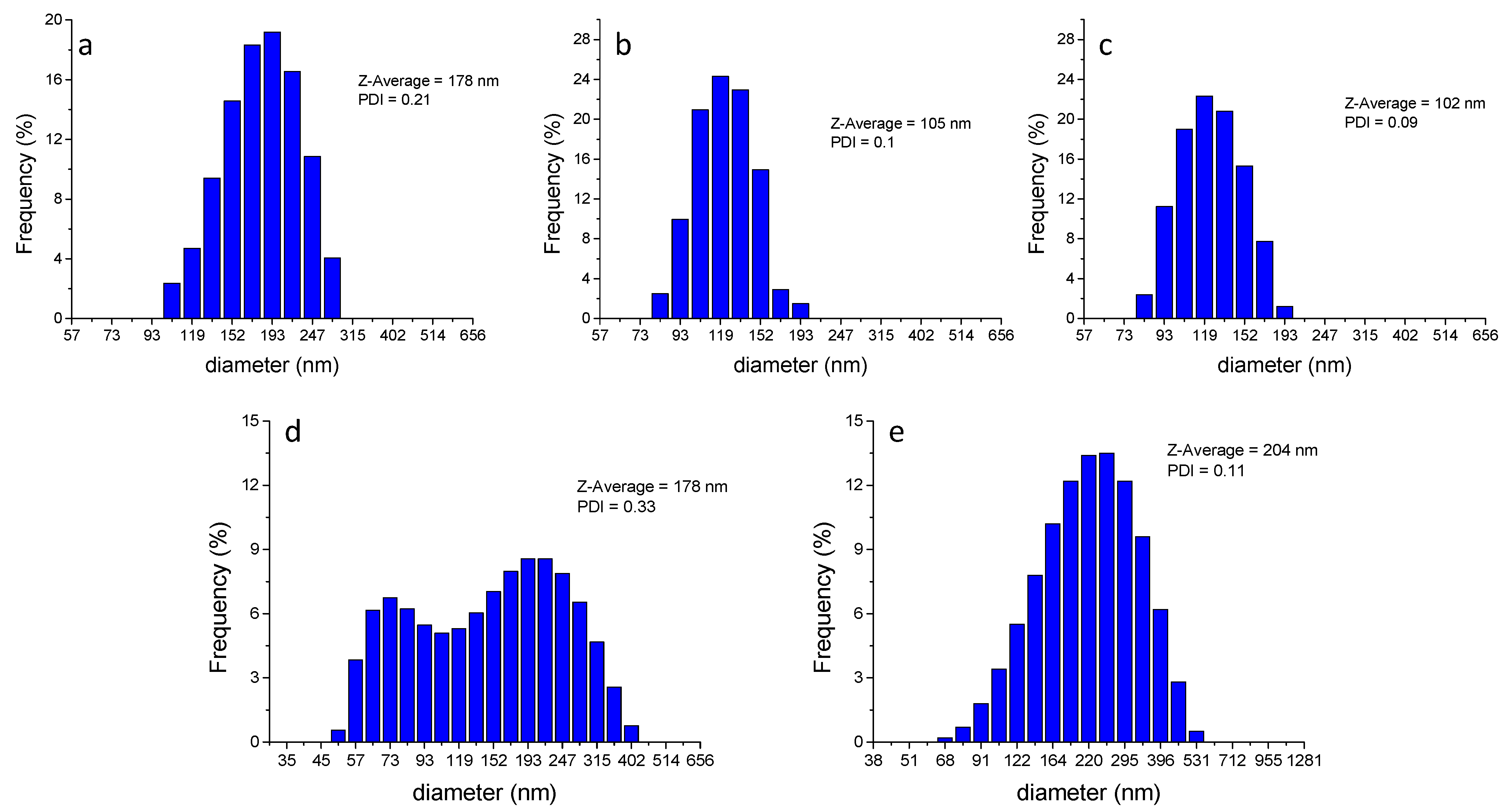

| Nanocarrier | calc. ET(30) a (kcal mol−1) | Calculated f(η2, εS) b | n | Calculated ε | Literature Values [3,30,31,32,33,34,35] | ||
|---|---|---|---|---|---|---|---|
| ε | ET(30) (kcal mol−1) | ||||||
| PSNP | neutral | 31.2 | −0.006 | 1.66 | 2.7 | 2.6 | n.a. * |
| aminated | 31.9 | 0.010 | 1.66 | 2.9 | |||
| carboxylated | 31.4 | −0.001 | 1.66 | 2.8 | |||
| PMMANPs | 35.0 | 0.034 | 1.55 | 3.5 | 3.5 | 35.6 | |
| PLGANPs | 36.2 | 0.054 | 1.49 | 3.8 | 3.0 | 35.8 | |
| BSA | 27.5 | −0.085 | 1.602 | 1.8 | n.a. * | 46.0 | |
| Pluronic P123 micelles | 38.1 | −0.098 | 1.46 | 4.8 | 42.0 | ||
| Tween 80 micelles | 38.3 | 0.100 | 1.47 | 5.1 | 55.0 | ||
References
- Fumagalli, L.; Ferrari, G.; Sampietro, M.; Gomila, G. Quantitative Nanoscale Dielectric Microscopy of Single-Layer Supported Biomembranes. Nano Lett. 2009, 9, 1604–1608. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.; Arinero, R.; Tordjeman, P.; Lévêque, G.; Schwartz, G.A.; Alegria, A.; Colmenero, J. Nanodielectric mapping of a model polystyrene-poly(vinyl acetate) blend by electrostatic force microscopy. Phys. Rev. E 2010, 81, 010801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, C.M.; Riley, E.A.; Palos-Chávez, J.; Reid, P.J. Measuring the Spatial Distribution of Dielectric Constants in Polymers through Quasi-Single Molecule Microscopy. J. Phys. Chem. B 2013, 117, 7106–7112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serghei, A.; Tress, M.; Kremer, F. Confinement Effects on the Relaxation Time Distribution of the Dynamic Glass Transition in Ultrathin Polymer Films. Macromolecules 2006, 39, 9385–9387. [Google Scholar] [CrossRef]
- Hou, Y.W.; Bardo, A.M.; Martinez, C.; Higgins, D.A. Characterization of Molecular Scale Environments in Polymer Films by Single Molecule Spectroscopy. J. Phys. Chem. B 2000, 104, 212–219. [Google Scholar] [CrossRef]
- Levitsky, I.; Krivoshlykov, S.G.; Grate, J.W. Rational Design of a Nile Red/Polymer Composite Film for Fluorescence Sensing of Organophosphonate Vapors Using Hydrogen Bond Acidic Polymers. Anal. Chem. 2001, 73, 3441–3448. [Google Scholar] [CrossRef]
- Yadigarli, A.; Song, Q.; Druzhinin, S.I.; Schönherr, H. Probing of local polarity in poly(methyl methacrylate) with the charge transfer transition in Nile red. Beilstein J. Org. Chem. 2019, 15, 2552–2562. [Google Scholar] [CrossRef] [Green Version]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem. Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Sackett, D.L.; Wolff, J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. J. Anal. Biochem. 1987, 167, 228–234. [Google Scholar] [CrossRef]
- Levitt, J.A.; Chung, P.-H.; Suhling, K. Spectrally resolved fluorescence lifetime imaging of Nile red for measurements of intracellular polarity. J. Biomed. Opt. 2015, 20, 096002. [Google Scholar] [CrossRef] [Green Version]
- Jee, A.Y.; Park, S.; Kwon, H.; Lee, M. Excited state dynamics of Nile Red in polymers. Chem. Phys. Lett. 2009, 477, 112–115. [Google Scholar] [CrossRef]
- Dias, L.C.; Custodio, R.; Pessine, F.B.T. Investigation of the Nile Red spectra by semi-empirical calculations and spectrophotometric measurements. Int. J. Quantum Chem. 2006, 106, 2624–2632. [Google Scholar] [CrossRef]
- Krishna, M.M. Excited-State Kinetics of the Hydrophobic Probe Nile Red in Membranes and Micelles. J. Phys. Chem. A 1999, 103, 3589–3595. [Google Scholar] [CrossRef]
- Hazra, P.; Chakrabarty, D.; Chakraborty, A.; Sarkar, N. Intramolecular charge transfer and solvation dynamics of Nile Red in the nanocavity of cyclodextrins. Chem. Phys. Lett. 2004, 388, 150–157. [Google Scholar] [CrossRef]
- Renge, I.J. Refractive index dependence of solvatochromism. Photochem. Photobiol. A 2018, 353, 433–444. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Y.; Xu, Y.; Qian, X. Naphthalimides for labeling and sensing applications. Pure Appl. Chem. 2014, 86, 1237–1246. [Google Scholar] [CrossRef]
- Christopherson, C.J.; Paisley, N.R.; Xiao, Z.; Algar, W.R.; Hudson, Z.M. Red-Emissive Cell-Penetrating Polymer Dots Exhibiting Thermally Activated Delayed Fluorescence for Cellular Imaging. J. Am. Chem. Soc. 2021, 143, 13342–13349. [Google Scholar] [CrossRef]
- Jena, S.; Dhanalakshmi, P.; Bano, G.; Thilagar, P. Delayed Fluorescence, Room Temperature Phosphorescence, and Mechanofluorochromic Naphthalimides: Differential Imaging of Normoxia and Hypoxia Live Cancer Cells. J. Phys. Chem. B 2020, 124, 5393–5406. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Y.; Liu, K.; Yu, X.; Wu, B.; Wu, H.; Gong, Z.; Yi, T. Iridium Complex Triggered White-Light-Emitting Gel and its Response to Cysteine. J. Mater. Chem. 2012, 22, 2650–2657. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, Y.; Sun, T.; Zhu, Y.; Ren, H.; Zou, X.; Ma, Y.; Meihaus, K.R.; Long, J.R.; Zhu, G. A Crystalline Polyimide Porous Organic Framework for Selective Adsorption of Acetylene over Ethylene. J. Am. Chem. Soc. 2018, 140, 15724–15730. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.B.; Phung, T.K.; Tran, T.T.T.; Mugemana, C.; Giang, H.N.; Nhi, T.L.P. Polystyrene nanoparticles prepared by nanoprecipitation: A recyclable template for fabricating hollow silica. J. Ind. Eng. Chem. 2021, 97, 307–315. [Google Scholar] [CrossRef]
- Aubrey, J.; Ganachaud, F.; Addad, J.P.C.; Cabane, B. Nanoprecipitation of Polymethylmethacrylate by Solvent Shifting: 1. Boundaries. Langmuir 2009, 25, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Formulation of functionalized PLGA polymeric nanoparticles for targeted drug delivery. Polymer 2015, 68, 41–46. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Avó, J.; Diniz, A.M.; Pinto, S.N.; Barbosa, J.; Smith, P.O.; Berberan-Santos, M.N.; Pålsson, L.O.; Dias, F.B. TADF Dye-Loaded Nanoparticles for Fluorescence Live-Cell Imaging. Front. Chem. 2020, 8, 404. [Google Scholar] [CrossRef]
- Olmsted, J., III. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, Y.; Wang, X.; Wang, Q.; Xu, W. Synthesis and Photochemical Properties of Novel 4-Diarylamine-1,8-naphthalimide Derivatives. Dye. Pigment. 2008, 77, 125–128. [Google Scholar] [CrossRef]
- Tanaka, H.; Shizu, K.; Nakanotani, H.; Adachi, C. Twisted Intramolecular Charge Transfer State for Long-Wavelength Thermally Activated Delayed Fluorescence. Chem. Mater. 2013, 25, 3766–3771. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, R.; Zhao, J.; Li, C. Spin-Orbit Charge Transfer Intersystem Crossing in Perylenemonoimide-Phenothiazine Compact Electron Donor-Acceptor Dyads. Chem. Commun. 2018, 54, 12329–12332. [Google Scholar] [CrossRef]
- Cerón-Carrasco, J.P.; Jacquemin, D.; Laurence, C.; Planchat, A.; Reichardt, C.; Sraïdi, K. Solvent polarity scales: Determination of new ET(30) values for 84 organic solvents. J. Phys. Org. Chem. 2014, 27, 512–518. [Google Scholar] [CrossRef]
- Ghosh, S.; Guchhait, N. Chemically Induced Unfolding of Bovine Serum Albumin by Urea and Sodium Dodecyl Sulfate: A Spectral Study with the Polarity-Sensitive Charge-Transfer Fluorescent Probe (E)-3-(4-Methylaminophenyl)acrylic Acid Methyl Ester. ChemPhysChem 2009, 10, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Thomas, S.P.; Kuryan, S.; Isac, J. Mechanical properties of ceramic-polymer nanocomposites. Express Polym. Lett. 2009, 3, 177–189. [Google Scholar] [CrossRef]
- Gomes, A.J.; Assunção, R.M.N.; Filho, G.R.; Espreafico, E.M.; Machado, A.E.H. Preparation and Characterization of Poly(D,L-lactic-co-glycolic acid) Nanoparticles Containing 3-(Benzoxazol-2-yl)-7-(N,N-diethyl amino)chromen-2-one. J. Appl. Polym. Sci. 2007, 105, 964–972. [Google Scholar] [CrossRef]
- Mishra, J.; Swain, J.; Mishra, A.K. Molecular Level Understanding of Sodium Dodecyl Sulfate (SDS) Induced Sol-Gel Transition of Pluronic F127 Using Fisetin as a Fluorescent Molecular Probe. J. Phys. Chem. B 2018, 122, 181–193. [Google Scholar] [CrossRef]
- Paul, B.K.; Ghosh, N.; Mukherjee, S. Modulated Photophysics and Rotational-Relaxation Dynamics of Coumarin 153 in Nonionic Micelles: The Role of Headgroup Size and Tail Length of the Surfactants. RSC Adv. 2015, 5, 9381–9388. [Google Scholar] [CrossRef]
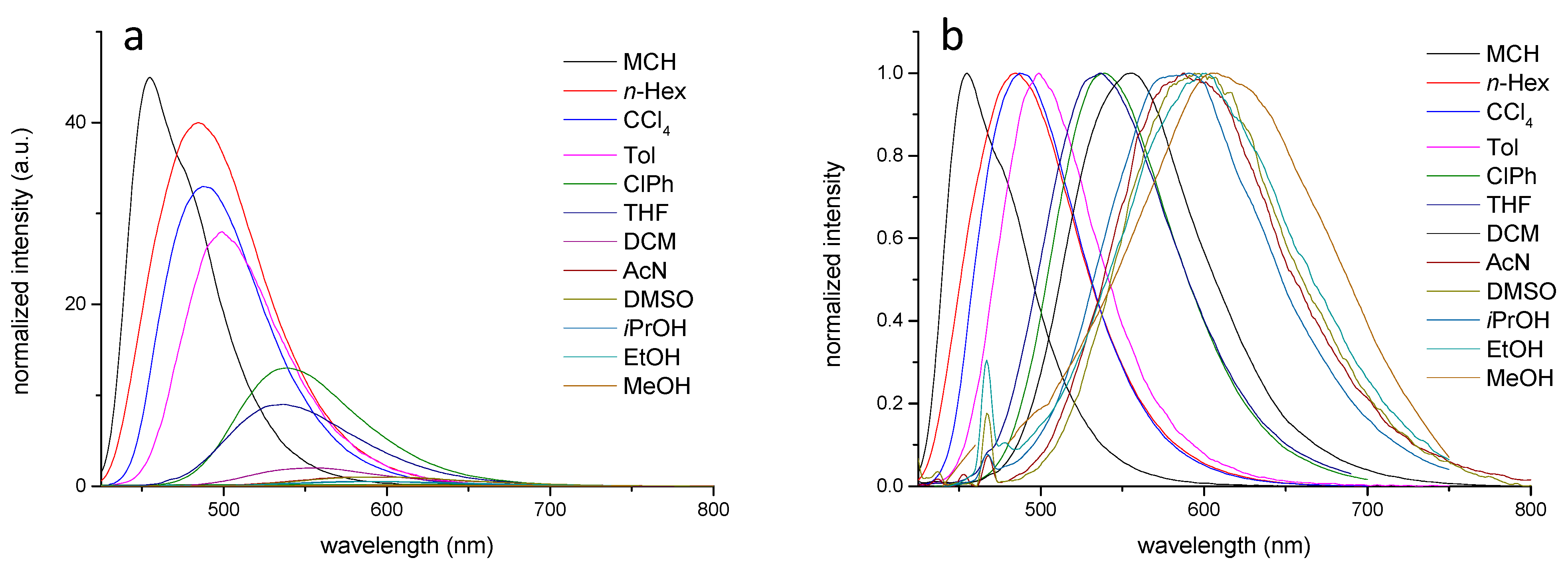
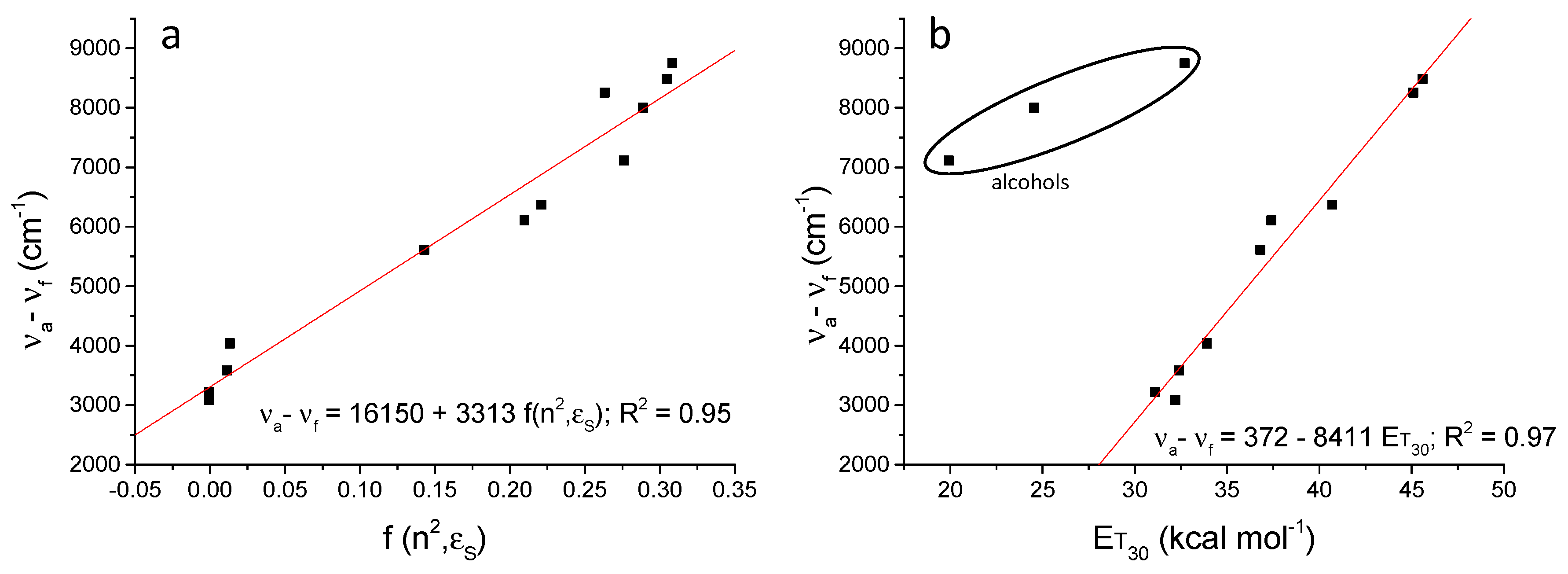

| Solvents a | υa (cm−1) | υf (cm−1) | Δυ (cm−1) | ΦF (%) b | τF (ns) | kr (ns−1) | knr (ns−1) |
|---|---|---|---|---|---|---|---|
| MCH | 24,096 | 21,008 | 3088 | 45.1 | 7.74 | 0.058 | 0.071 |
| n-Hexane | 23,753 | 20,534 | 3219 | 39.9 | 6.62 | 0.060 | 0.091 |
| CCl4 | 23,866 | 20,284 | 3582 | 33.3 | 7.80 | 0.043 | 0.087 |
| Toluene | 24,038 | 20,000 | 4038 | 28.1 | 7.43 | 0.038 | 0.097 |
| ClPh | 23,697 | 18,083 | 5613 | 13.0 | 9.24 | 0.014 | 0.094 |
| THF | 24,390 | 18,282 | 6108 | 9.1 | 6.38 | 0.014 | 0.142 |
| DCM | 24,038 | 17,668 | 6370 | 7.9 | 9.46 | 0.009 | 0.097 |
| AcN | 24,691 | 16,207 | 8484 | 2.3 | 4.01 | 0.005 | 0.245 |
| DMSO | 24,155 | 15,898 | 8256 | 1.0 | 2.69 | 0.004 | 0.367 |
| iPrOH | 23,753 | 16,639 | 7114 | 0.5 | 2.48 | 0.002 | 0.401 |
| EtOH | 23,697 | 15,699 | 7998 | 0.5 | 0.67 | 0.008 | 1.487 |
| MeOH | 23,923 | 15,175 | 8748 | 0.1 | 0.23 | 0.004 | 4.401 |
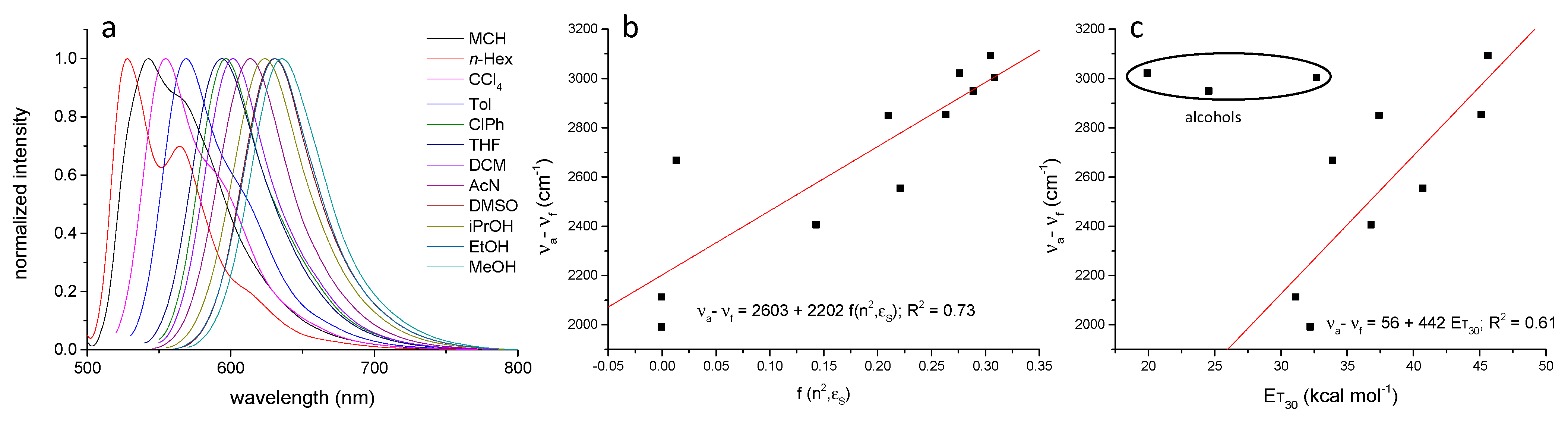
| Nanocarrier | υa a (cm−1) | υf (cm−1) | Δυ (cm−1) | ΦFf b (%) | τF (ns) | kr (ns−1) | knr (ns−1) | |
|---|---|---|---|---|---|---|---|---|
| PSNP | neutral | 24,113 | 20,703 | 3410 | 42.0 | 9.51 | 0.044 | 0.061 |
| aminated | 23,923 | 20,449 | 3474 | 38.1 | 10.71 | 0.035 | 0.058 | |
| carboxylated | 23,980 | 20,618 | 3362 | 39.2 | 11.63 | 0.033 | 0.052 | |
| PMMANPs | 23,952 | 20,080 | 3872 | 30.7 | 9.96 | 0.031 | 0.069 | |
| PLGANPs | 23,923 | 19,762 | 4161 | 24.9 | 11.18 | 0.022 | 0.067 | |
| BSA | 23,584 | 21,929 | 1655 | 62.6 | 5.14 | 0.123 | 0.072 | |
| Pluronic P123 micelles | 23,923 | 18,622 | 5301 | 12.1 | 9.74 | 0.012 | 0.090 | |
| Tween 80 micelles | 23,866 | 18,657 | 5209 | 12.9 | 6.02 | 0.022 | 0.145 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, A.M.; Cruz, R.P.R.; Avó, J. Probing the Local Polarity in Biocompatible Nanocarriers with Solvatofluorochromism of a 4-Carbazole-1,8-naphthalimide Dye. Photochem 2022, 2, 489-502. https://doi.org/10.3390/photochem2030034
Diniz AM, Cruz RPR, Avó J. Probing the Local Polarity in Biocompatible Nanocarriers with Solvatofluorochromism of a 4-Carbazole-1,8-naphthalimide Dye. Photochem. 2022; 2(3):489-502. https://doi.org/10.3390/photochem2030034
Chicago/Turabian StyleDiniz, Ana M., Rui P. R. Cruz, and João Avó. 2022. "Probing the Local Polarity in Biocompatible Nanocarriers with Solvatofluorochromism of a 4-Carbazole-1,8-naphthalimide Dye" Photochem 2, no. 3: 489-502. https://doi.org/10.3390/photochem2030034