g-C3N4/MoS2 Heterojunction for Photocatalytic Removal of Phenol and Cr(VI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of g-C3N4, MoS2 and Composite MoS2/g-C3N4 Photocatalysts
2.2.1. Synthesis of Bare Carbon Nitride (g-C3N4) and Molybdenum Disulfide (MoS2)
2.2.2. Synthesis of MoS2/g-C3N4 Heterostructure
2.3. Material Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
3.1. Characterization of the Prepared Photocatalysts
3.1.1. Structural Characterization
3.1.2. Morphology Surface Analysis
3.1.3. UV–Vis Spectroscopy and Band Gap Determination
3.2. Photocatalytic Study
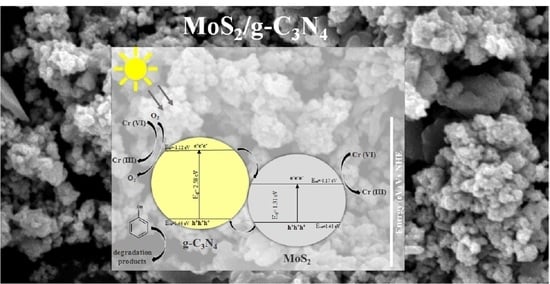
3.3. Photocatalytic Mechanism for the Composite Photocatalysts
3.4. Recyclability of the Composite Photocatalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez-Rangel, K.Y.; Lartundo-Rojas, L.; García-García, A.; Cipagauta-Díaz, S.; Mantilla, A.; Samaniego-Benítez, J.E. Hydrothermal Synthesis of a Two-Dimensional g-C3N4/MoS2/MnOOH Composite Material and Its Potential Application as Photocatalyst. J. Chem. Technol. Biotechnol. 2019, 94, 3447–3456. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Giannakas, A.; Konstantinou, I. Simultaneous Photocatalytic Reduction of Cr(VI) and Oxidation of Benzoic Acid in Aqueous N-F-Codoped TiOsuspensions: Optimization and Modeling Using the Response Surface Methodology. Int. J. Photoenergy 2012, 1, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, R.; Niu, S.; Zheng, J.; Guo, C. Enhanced Photo-Catalytic Performance by Effective Electron-Hole Separation for MoS2 Inlaying in g-C3N4 Hetero-Junction. Appl. Surf. Sci. 2019, 475, 355–362. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Díaz, C.; Cañizares, P.; Fernández, F.J.; Natividad, R.; Rodrigo, M.A.; Rosedal, E.; Toluca, E.; de México, M. Electrochemical Advanced Oxidation Processes: An Overview of the Current Applications to Actual Industrial Effluents. J. Mex. Chem. Soc. 2014, 58, 3. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of Thiourea into Carbon Nitride Semiconductors as Visible Light Photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I. WO3 Fibers/g-C3N4 Z-Scheme Heterostructure Photocatalysts for Simultaneous Oxidation/Reduction of Phenol/Cr(VI) in Aquatic Media. Catalysts 2021, 11, 792. [Google Scholar] [CrossRef]
- Sun, K.; Jia, F.; Yang, B.; Lin, C.; Li, X.; Song, S. Synergistic Effect in the Reduction of Cr(VI) with Ag-MoS2 as Photocatalyst. Appl. Mater. Today 2020, 18, 100453. [Google Scholar] [CrossRef]
- Sun, H.; Wu, T.; Zhang, Y.; Ng, D.H.L.; Wang, G. Structure-Enhanced Removal of Cr(VI) in Aqueous Solutions Using MoS2 Ultrathin Nanosheets. New. J. Chem. 2018, 42, 9006–9015. [Google Scholar] [CrossRef]
- Wang, K.; Chen, P.; Nie, W.; Xu, Y.; Zhou, Y. Improved Photocatalytic Reduction of Cr(VI) by Molybdenum Disulfide Modified with Conjugated Polyvinyl Alcohol. Chem. Eng. J. 2019, 359, 1205–1214. [Google Scholar] [CrossRef]
- Fageria, P.; Sudharshan, K.Y.; Nazir, R.; Basu, M.; Pande, S. Decoration of MoS2 on g-C3N4 Surface for Efficient Hydrogen Evolution Reaction. Electrochim. Acta 2017, 258, 1273–1283. [Google Scholar] [CrossRef]
- Qi, Y.; Liang, Q.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.H. Synthesis and Photocatalytic Activity of Mesoporous g-C3N4/MoS2 Hybrid Catalysts. R. Soc. Open Sci. 2018, 5, 180187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samy, O.; el Moutaouakil, A. A Review on MoS2 Energy Applications: Recent Developments and Challenges. Energies 2021, 14, 4568. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic Carbon Nitride (g-C3N4) Nanocomposites: A New and Exciting Generation of Visible Light Driven Photocatalysts for Environmental Pollution Remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, G.; Hou, Y.; Wang, X. Layering MoS2 on Soft Hollow g-C3N4 Nanostructures for Photocatalytic Hydrogen Evolution. Appl. Catal. A Gen. 2016, 521, 2–8. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Chen, Z.; Gong, S.; Huang, H. Surface Affinity Modulation of MoS2 by Hydrothermal Synthesis and Its Intermediary Function in Interfacial Chemistry. Chem. Phys. Lett. 2019, 730, 608–611. [Google Scholar] [CrossRef]
- Koutsouroubi, E.D.; Vamvasakis, I.; Papadas, I.T.; Drivas, C.; Choulis, S.A.; Kennou, S.; Armatas, G.S. Interface Engineering of MoS2-Modified Graphitic Carbon Nitride Nano-Photocatalysts for an Efficient Hydrogen Evolution Reaction. ChemPlusChem 2020, 85, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Sivasankaran, R.P.; Rockstroh, N.; Kreyenschulte, C.R.; Bartling, S.; Lund, H.; Acharjya, A.; Junge, H.; Thomas, A.; Brückner, A. Influence of MoS2 on Activity and Stability of Carbon Nitride in Photocatalytic Hydrogen Production. Catalysts 2019, 9, 695. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Zhang, Q.; Zhang, Y.; Yang, Z.; Zhu, A.; Dionysiou, D.D. Enhancement of the Cr(VI) Adsorption and Photocatalytic Reduction Activity of g-C3N4 by Hydrothermal Treatment in HNO3 Aqueous Solution. Appl. Catal. A Gen. 2016, 521, 9–18. [Google Scholar] [CrossRef]
- Tian, Y.; Ge, L.; Wang, K.; Chai, Y. Synthesis of Novel MoS2/g-C3N4 Heterojunction Photocatalysts with Enhanced Hydrogen Evolution Activity. Mater. Charact. 2014, 87, 70–73. [Google Scholar] [CrossRef]
- Visic, B.; Dominko, R.; Gunde, M.K.; Hauptman, N.; Skapin, S.D.; Remskar, M. Optical Properties of Exfoliated MoS2 Coaxial Nanotubes—Analogues of Graphene. Nanoscale Res. Lett. 2011, 6, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.H.; Hu, X.G.; Wang, J.; Xu, Y.F.; Han, C.L. Tribological Properties of MoS2 with Different Morphologies in High-Density Polyethylene. Tribol. Lett. 2012, 47, 79–90. [Google Scholar] [CrossRef]
- Konstas, P.S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts 2018, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Amini, M.; Ahmad Ramazani, S.A.; Faghihi, M.; Fattahpour, S. Preparation of Nanostructured and Nanosheets of MoS2 Oxide Using Oxidation Method. Ultrason. Sonochem. 2017, 39, 188–196. [Google Scholar] [CrossRef]
- Zhang, X.; Suo, H.; Zhang, R.; Niu, S.; Zhao, X.; Zheng, J.; Guo, C. Photocatalytic Activity of 3D Flower-like MoS2 Hemispheres. Mater. Res. Bull. 2018, 100, 249–253. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, E.; Ma, Y.; Hu, X.; Wan, J.; Sun, L.; Fan, J. Synthesis of MoS2/g-C3N4 Nanosheets as 2D Heterojunction Photocatalysts with Enhanced Visible Light Activity. Appl. Surf. Sci. 2016, 364, 694–702. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A Review of Chemical, Electrochemical and Biological Methods for Aqueous Cr(VI) Reduction. J. Hazard. Mater. 2012, 224, 1–12. [Google Scholar] [CrossRef]
- Du, X.; Yi, X.; Wang, P.; Deng, J.; Wang, C.C. Enhanced Photocatalytic Cr(VI) Reduction and Diclofenac Sodium Degradation under Simulated Sunlight Irradiation over MIL-100(Fe)/g-C3N4 Heterojunctions. Cuihua Xuebao/Chin. J. Catal. 2019, 40, 70–79. [Google Scholar] [CrossRef]










| Catalyst | Specific Surface Area SBET (m2g−1) | Average Pore Diameter (nm) | VTOT (cm3 g−1) |
|---|---|---|---|
| MoS2 | 2.0 | 31 | 0.016 |
| g-C3N4 | 81.3 | 8.3 | 0.169 |
| 0.5% MoS2/g-C3N4 | 93.7 | 10.2 | 0.238 |
| 1% MoS2/g-C3N4 | 62.2 | 9.5 | 0.148 |
| 3% MoS2/g-C3N4 | 90.2 | 10.9 | 0.245 |
| 10% MoS2/g-C3N4 | 92.1 | 10.5 | 0.239 |
| Catalyst | Energy Band Gap (eV) | Absorption Edge λ (nm) |
|---|---|---|
| g-C3N4 | 2.58 | 480 |
| MoS2 | 1.31 | 946 |
| 0.5% MoS2/g-C3N4 | 2.56 | 484 |
| 1% MoS2/g-C3N4 | 2.66 | 466 |
| 3% MoS2/g-C3N4 | 2.64 | 469 |
| 10% MoS2/g-C3N4 | 2.64 | 469 |
| Catalyst | k (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| g-C3N4 | 0.022 | 31.5 | 0.9401 |
| MoS2 | 0.0002 | 3465.0 | 0.5016 |
| 0.5MoS2/g-C3N4 | 0.006 | 116.0 | 0.9528 |
| 1MoS2/g-C3N4 | 0.009 | 77.0 | 0.9940 |
| 3MoS2/g-C3N4 | 0.005 | 139.0 | 0.9250 |
| 10MoS2/g-C3N4 | 0.006 | 116.0 | 0.9158 |
| Binary | Phenol | Cr(VI) | ||||
|---|---|---|---|---|---|---|
| Catalyst | k (min−1) | t1/2 (min) | R2 | k (min−1) | t1/2 (min) | R2 |
| g-C3N4 | 0.068 | 10.2 | 0.9978 | 0.003 | 231.0 | 0.9477 |
| MoS2 | 0.002 | 346.5 | 0.9373 | 0.002 | 346.5 | 0.9694 |
| 0.5MoS2/g-C3N4 | 0.042 | 16.5 | 0.9738 | 0.002 | 346.5 | 0.9552 |
| 1MoS2/g-C3N4 | 0.091 | 7.6 | 0.9246 | 0.003 | 231.0 | 0.9740 |
| 3MoS2/g-C3N4 | 0.042 | 16.5 | 0.9513 | 0.002 | 346.5 | 0.9478 |
| 10MoS2/g-C3N4 | 0.031 | 22.4 | 0.9374 | 0.003 | 231.0 | 0.9325 |
| Scavenger | k (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| IPA | 0.003 | 231 | 0.9742 |
| SOD | 0.004 | 173.2 | 0.9590 |
| TEOA | 0.002 | 346.5 | 0.9553 |
| no scavenger | 0.009 | 77.0 | 0.9940 |
| Cycle | k (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| 1 | 0.09 | 7.7 | 0.9510 |
| 2 | 0.089 | 7.8 | 0.9453 |
| 3 | 0.083 | 8.3 | 0.9496 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapti, I.; Bairamis, F.; Konstantinou, I. g-C3N4/MoS2 Heterojunction for Photocatalytic Removal of Phenol and Cr(VI). Photochem 2021, 1, 358-370. https://doi.org/10.3390/photochem1030023
Rapti I, Bairamis F, Konstantinou I. g-C3N4/MoS2 Heterojunction for Photocatalytic Removal of Phenol and Cr(VI). Photochem. 2021; 1(3):358-370. https://doi.org/10.3390/photochem1030023
Chicago/Turabian StyleRapti, Ilaeira, Feidias Bairamis, and Ioannis Konstantinou. 2021. "g-C3N4/MoS2 Heterojunction for Photocatalytic Removal of Phenol and Cr(VI)" Photochem 1, no. 3: 358-370. https://doi.org/10.3390/photochem1030023