Antioxidant Enzyme Activities as Biomarkers of Cu and Pb Stress in Centella asiatica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Hydroponic Design
2.2. Metal Analysis
2.3. Analysis of Antioxidant Enzyme Activities
2.4. Statistical Analysis
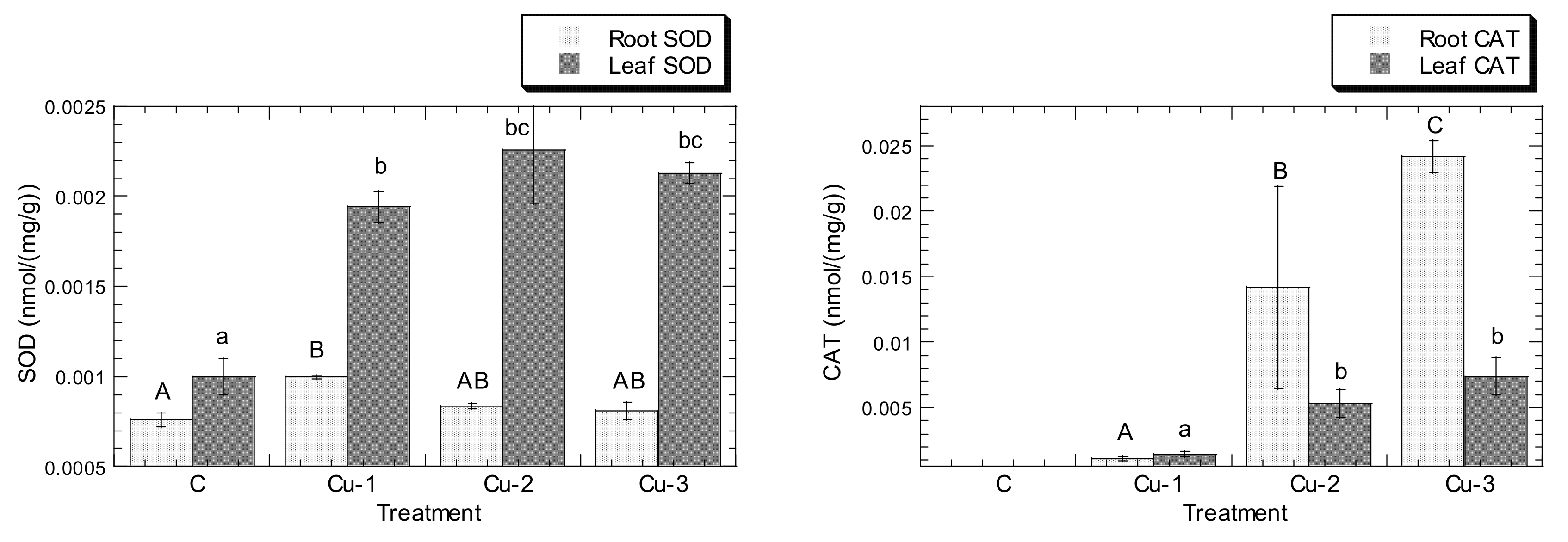
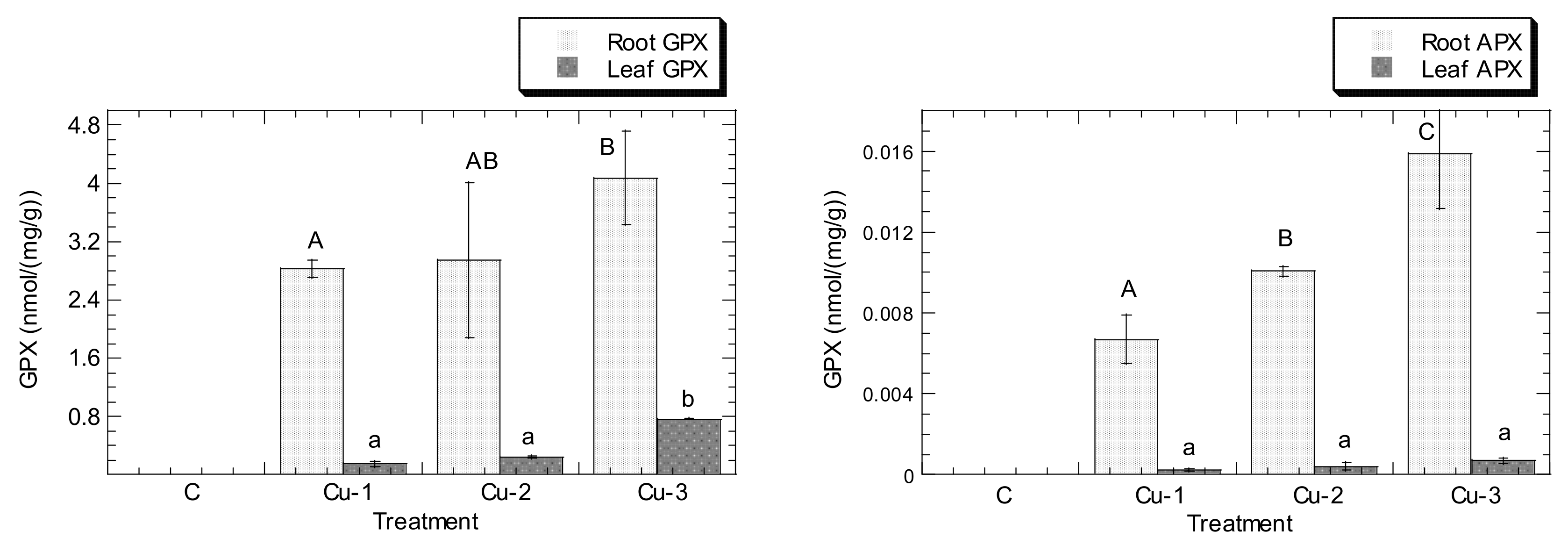
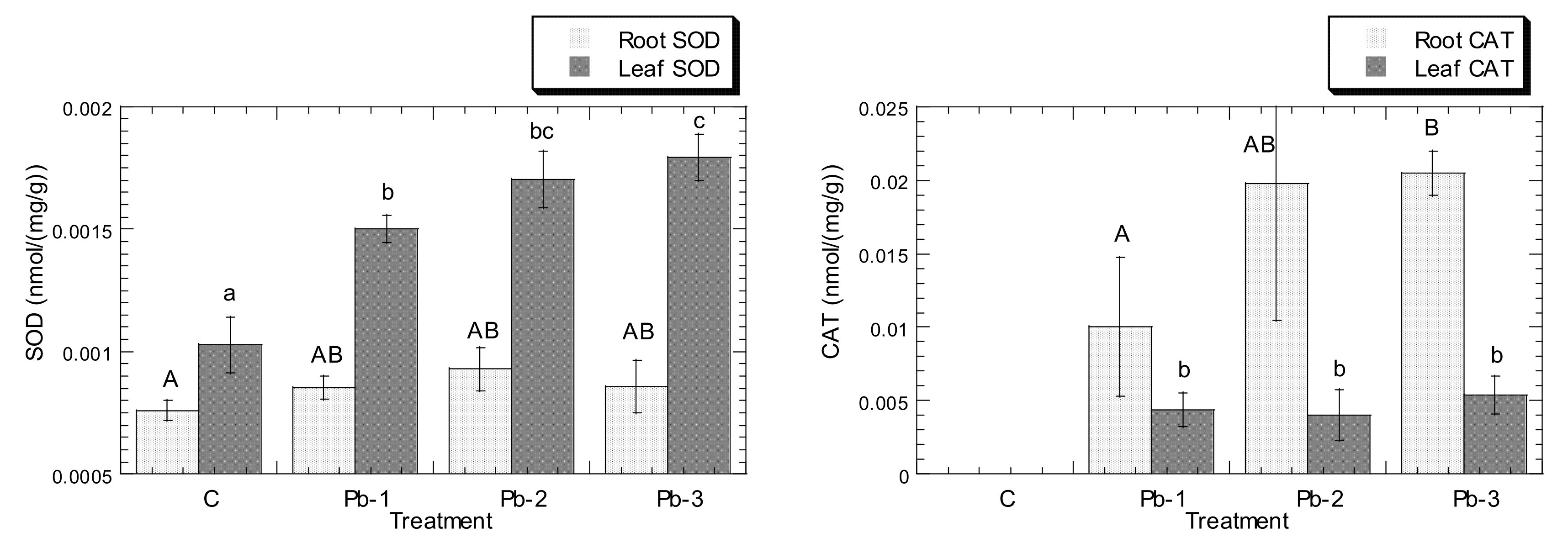
3. Results
4. Discussion
4.1. Higher Accumulations of Cu and Pb in the Roots Than in the Leaves
4.2. Synergistic Effects of Cu and Pb Stress at Higher Metal-Level Exposures
4.3. Cu and Pb Stress Triggered the Increment of APX, CAT, GPX, and SOD Levels in Both Leaves and Roots
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Israr, M.; Jewell, A.; Kumar, D.; Sahi, S.V. Interactive Effects of Lead, Copper, Nickel and Zinc on Growth, Metal Uptake and Antioxidative Metabolism of Sesbania drummondii. J. Hazard. Mater. 2011, 186, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Samal, S. Alternaria Host-Specific (HSTs) Toxins: An Overview of Chemical Characterization, Target Sites, Regulation and Their Toxic Effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- Kohmoto, K.; Otani, H. Host Recognition by Toxigenic Plant Pathogens. Experientia 1991, 47, 755–764. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zou, J.; Jiang, W.; Liu, D. Effect of Cu2+ Concentration on Growth, Antioxidant Enzyme Activity and Malondialdehyde Content in Garlic (Allium sativum L.). Acta Biol. Cracoviensia Ser. Bot. 2007, 49, 95–101. [Google Scholar]
- Hegedüs, A.; Erdei, S.; Horváth, G. Comparative Studies of H2O2 Detoxifying Enzymes in Green and Greening Barley Seedlings under Cadmium Stress. Plant Sci. 2001, 160, 1085–1093. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S.K. Plants as Natural Antioxidants. Nat. Prod. Radiance 2006, 5, 9. [Google Scholar]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Lamhamdi, M.; Bakrim, A.; Aarab, A.; Lafont, R.; Sayah, F. Lead Phytotoxicity on Wheat (Triticum aestivum L.) Seed Germination and Seedlings Growth. Comptes Rendus Biol. 2011, 334, 118–126. [Google Scholar] [CrossRef]
- Tang, L.; Kwon, S.-Y.; Kim, S.-H.; Kim, J.-S.; Choi, J.S.; Cho, K.Y.; Sung, C.K.; Kwak, S.-S.; Lee, H.-S. Enhanced Tolerance of Transgenic Potato Plants Expressing Both Superoxide Dismutase and Ascorbate Peroxidase in Chloroplasts against Oxidative Stress and High Temperature. Plant Cell Rep. 2006, 25, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Mohd Nadzir, M. Comparative Studies on Different Extraction Methods of Centella asiatica and Extracts Bioactive Compounds Effects on Antimicrobial Activities. Antibiotics 2021, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Seevaratnam, V.; Banumathi, P.; Premalatha, M.R.; Sundaram, S.; Arumugam, T. Functional Properties of Centella asiatica (L.): A Review. Int. J. Pharm. Pharm. Sci. 2012, 4, 8–14. [Google Scholar]
- Sarumathi, A.; Anbu, S.; Saravanan, N. Antibacterial Activity of Centella asiatica (Linn.) Leaves. Int. J. Chem. Pharm. Sci. 2013, 1, 393–395. [Google Scholar]
- Yap, C.K.; Fitri, M.; Yazdani, M.; Tan, S. Effects of Metal-Contaminated Soils on the Accumulation of Heavy Metals in Different Parts of Centella asiatica: A Laboratory Study. Sains Malays. 2010, 39, 347–352. [Google Scholar]
- Ong, G.H.; Yap, C.K.; Maziah, M.; Tan, S. Synergistic and Antagonistic Effects of Zinc Bioaccumulation with Lead and Antioxidant Activities in Centella asiatica. Sains Malays. 2013, 42, 1549–1555. [Google Scholar]
- Ong, G.H.; Yap, C.K.; Marziah, M.; Tan, S. Accumulation of Heavy Metals and Antioxidative Enzymes of Centella asiatica in Relation to Metals of the Soils. Pertanika J. Trop. Agric. Sci. 2013, 36, 331–336. [Google Scholar]
- Ong, G.H.; Yap, C.K.; Maziah, M.; Tan, S.G. The Effect of Cu Exposure on the Bioaccumulation of Zn and Antioxidant Activities in Different Parts of Centella asiatica. Asian J. Microbiol. Biotechnol. Environ. Sci. 2011, 13, 387–392. [Google Scholar]
- Yap, C.K.; Ong, G.H.; Cheng, W.H.; Nulit, R.; Ali, K.; Al-Shami, S.A. Interactive effects of copper and lead on metal uptake and antoixidative metabolism of Centella asiatica under experimental hydroponic systems. In Advances in Hydroponics Research; Webster, D.J., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 57–72. [Google Scholar]
- Tang, Y.-T.; Qiu, R.-L.; Zeng, X.-W.; Ying, R.-R.; Yu, F.-M.; Zhou, X.-Y. Lead, Zinc, Cadmium Hyperaccumulation and Growth Stimulation in Arabis paniculata Franch. Environ. Exp. Bot. 2009, 66, 126–134. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Blamey, F.P.C.; Asher, C.J.; Menzies, N.W. Trace Metal Phytotoxicity in Solution Culture: A Review. J. Exp. Bot. 2010, 61, 945–954. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Long, X.; Ni, W.; Fu, C. Sedum Alfredii H: A New Zn Hyperaccumulating Plant First Found in China. Chin. Sci. Bull. 2002, 47, 1634–1637. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N.V. Phytochelatin Synthesis and Response of Antioxidants during Cadmium Stress in Bacopa monnieri L. Plant Physiol. Biochem. PPB 2006, 44, 25–37. [Google Scholar] [CrossRef]
- Hemeda, H.M.; Klein, B.P. Effects of Naturally Occurring Antioxidants on Peroxidase Activity of Vegetable Extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Baxter, I.R.; Richards, E.L.; Freeman, J.L.; Murphy, A.S. Phytoremediation and hyperaccumulator plants. In Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man; Tamas, M.J., Martinoia, E., Eds.; Topics in Current Genetics; Springer: Berlin/Heidelberg, Germany, 2006; pp. 299–340. ISBN 978-3-540-31719-7. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-Driven Removal of Heavy Metals from Soil: Uptake, Translocation, Tolerance Mechanism, Challenges, and Future Perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar] [CrossRef]
- Tong, Y.P.; Kneer, R.; Zhu, Y.G. Vacuolar Compartmentalization: A Second-Generation Approach to Engineering Plants for Phytoremediation. Trends Plant Sci. 2004, 9, 7–9. [Google Scholar] [CrossRef]
- An, Y.-J. Assessment of Comparative Toxicities of Lead and Copper Using Plant Assay. Chemosphere 2006, 62, 1359–1365. [Google Scholar] [CrossRef]
- Nicholls, A.M.; Mal, T.K. Effects of Lead and Copper Exposure on Growth of an Invasive Weed, Lythrum salicaria L. (Purple Loosestrife). Ohio J. Sci. 2003, 103, 129–133. [Google Scholar]
- Alloway, B.J. Soil Factors Associated with Zinc Deficiency in Crops and Humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-0-8493-1575-6. [Google Scholar]
- Manan, F.A.; Mamat, D.D.; Samad, A.A.; Ong, Y.S.; Ooh, K.F.; Chai, T.T. Heavy Metal Accumulation and Antioxidant Properties of Nephrolepis biserrata Growing in Heavy Metal-Contaminated Soil. Glob. NEST J. 2015, 17, 544–554. [Google Scholar]
- Nadgórska-Socha, A.; Kafel, A.; Kandziora-Ciupa, M.; Gospodarek, J.; Zawisza-Raszka, A. Accumulation of Heavy Metals and Antioxidant Responses in Vicia faba Plants Grown on Monometallic Contaminated Soil. Environ. Sci. Pollut. Res. Int. 2013, 20, 1124–1134. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.M.; Kumar, S.G.; Jyothsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead Induced Changes in Antioxidant Metabolism of Horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and Bengalgram (Cicer arietinum L.). Chemosphere 2005, 60, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase Function in Plants: A Focus on Arabidopsis Mutants as Stress-Mimic Models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kafel, A.; Nadgórska-Socha, A.; Gospodarek, J.; Babczyńska, A.; Skowronek, M.; Kandziora, M.; Rozpedek, K. The Effects of Aphis fabae Infestation on the Antioxidant Response and Heavy Metal Content in Field Grown Philadelphus coronarius Plants. Sci. Total Environ. 2010, 408, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Panda, S.K. Zinc Reduces Copper Toxicity Induced Oxidative Stress by Promoting Antioxidant Defense in Freshly Grown Aquatic Duckweed Spirodela polyrhiza L. J. Hazard. Mater. 2010, 175, 1081–1084. [Google Scholar] [CrossRef]
- Guo, T.R.; Zhang, G.P.; Zhang, Y.H. Physiological Changes in Barley Plants under Combined Toxicity of Aluminum, Copper and Cadmium. Colloids Surf. B Biointerfaces 2007, 57, 182–188. [Google Scholar] [CrossRef]




| No | Treatments | Metal Concentrations |
|---|---|---|
| 1 | Control | No metals added |
| 2 | Cu-1 | Cu (0.10) |
| 3 | Cu-2 | Cu (0.20) |
| 4 | Cu-3 | Cu (0.30) |
| 5 | Pb-1 | Pb (0.20) |
| 6 | Pb-2 | Pb (0.40) |
| 7 | Pb-3 | Pb (0.60) |
| 8 | CuPb-1 | Cu (0.10) + Pb (0.20) |
| 9 | CuPb-2 | Cu (0.20) + Pb (0.40) |
| 10 | CuPb-3 | Cu (0.30) + Pb (0.60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, C.K.; Tan, W.S.; Wong, K.W.; Ong, G.H.; Cheng, W.H.; Nulit, R.; Ibrahim, M.H.; Chew, W.; Berandah Edward, F.; Okamura, H.; et al. Antioxidant Enzyme Activities as Biomarkers of Cu and Pb Stress in Centella asiatica. Stresses 2021, 1, 253-265. https://doi.org/10.3390/stresses1040018
Yap CK, Tan WS, Wong KW, Ong GH, Cheng WH, Nulit R, Ibrahim MH, Chew W, Berandah Edward F, Okamura H, et al. Antioxidant Enzyme Activities as Biomarkers of Cu and Pb Stress in Centella asiatica. Stresses. 2021; 1(4):253-265. https://doi.org/10.3390/stresses1040018
Chicago/Turabian StyleYap, Chee Kong, Wen Siang Tan, Koe Wei Wong, Ghim Hock Ong, Wan Hee Cheng, Rosimah Nulit, Mohd. Hafiz Ibrahim, Weiyun Chew, Franklin Berandah Edward, Hideo Okamura, and et al. 2021. "Antioxidant Enzyme Activities as Biomarkers of Cu and Pb Stress in Centella asiatica" Stresses 1, no. 4: 253-265. https://doi.org/10.3390/stresses1040018






