A Brief Overview on Preparation of Self-Healing Polymers and Coatings via Hydrogen Bonding Interactions
Abstract
:1. Introduction
2. Hydrogen Bonding
3. Self-Healing Material via H-Bonding Interactions
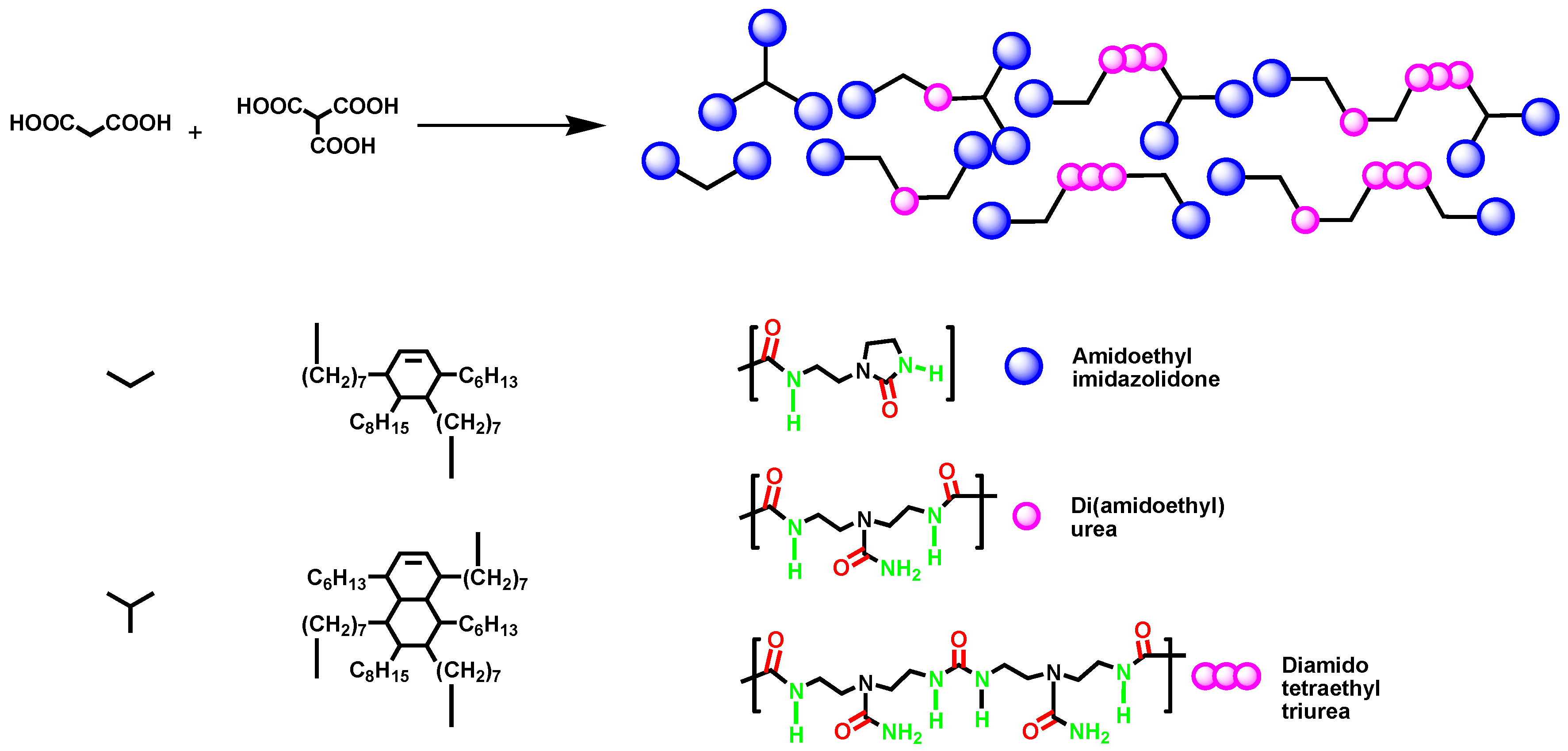
3.1. Self-Healing Using 2-Aminoethyl-Imidazolidone (UDETA)
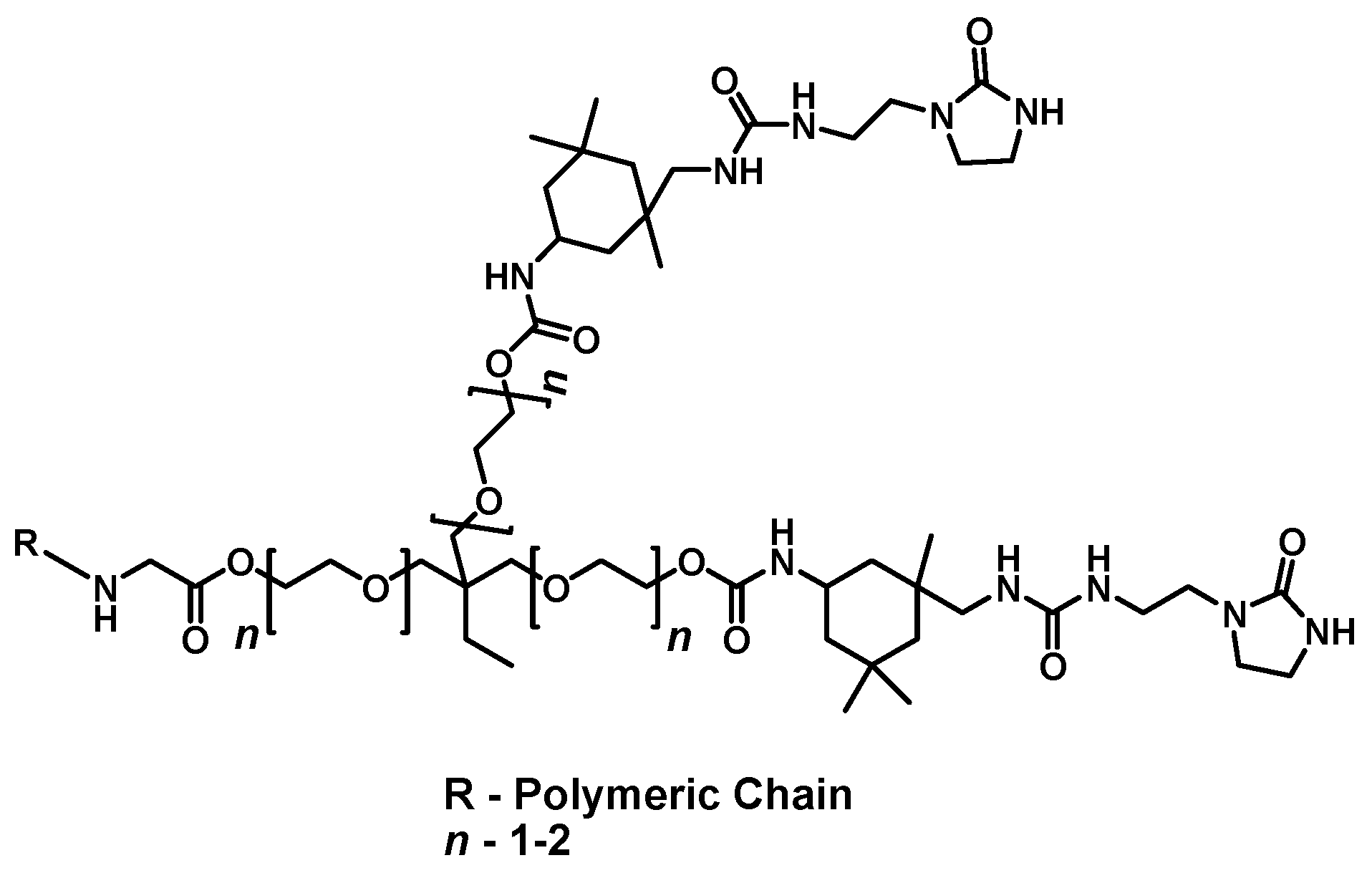
3.2. Moisture-Mediated Self-Healing Coating
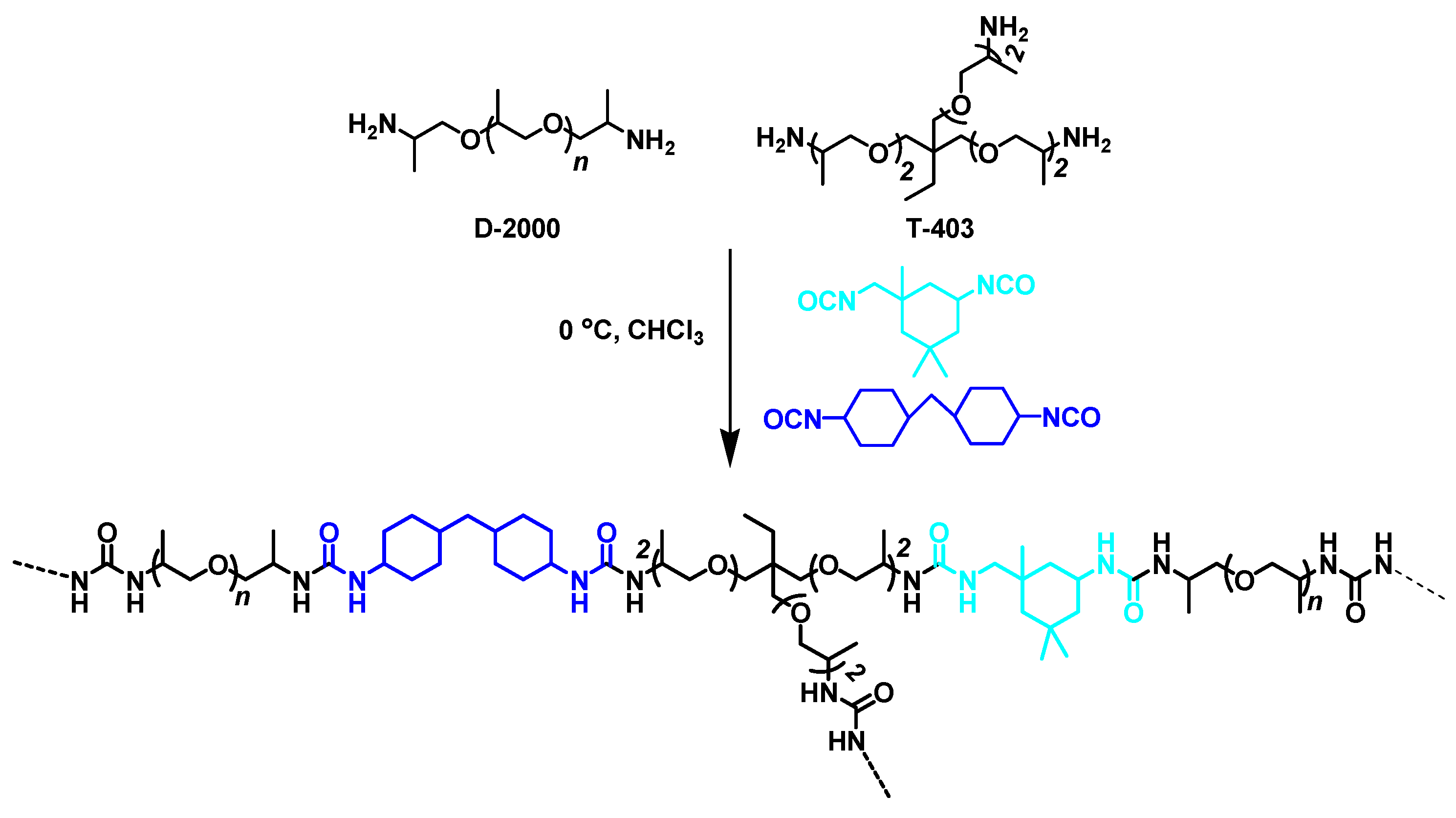
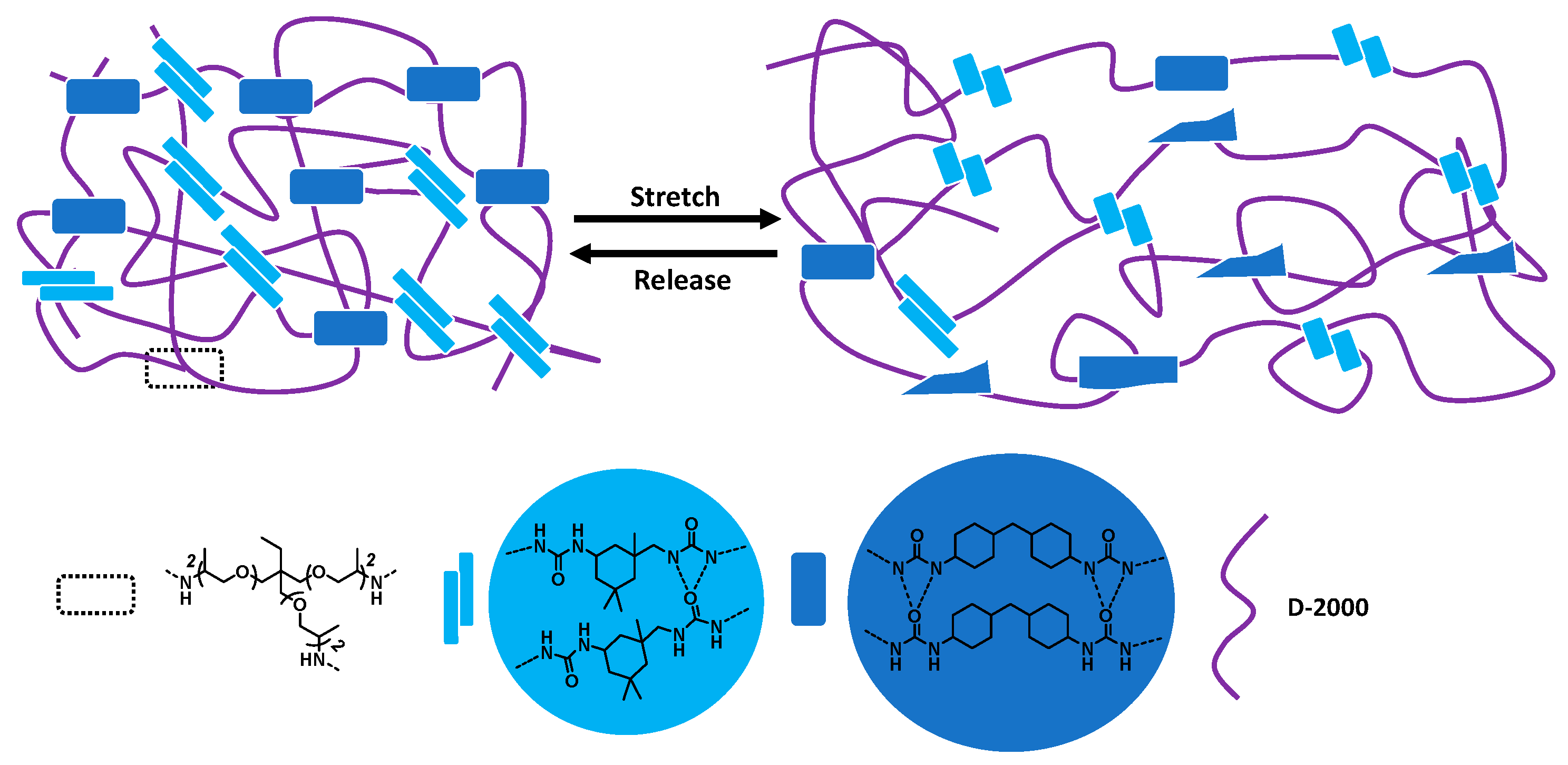
3.3. Trigger Free Self-Healable Brush Polymer
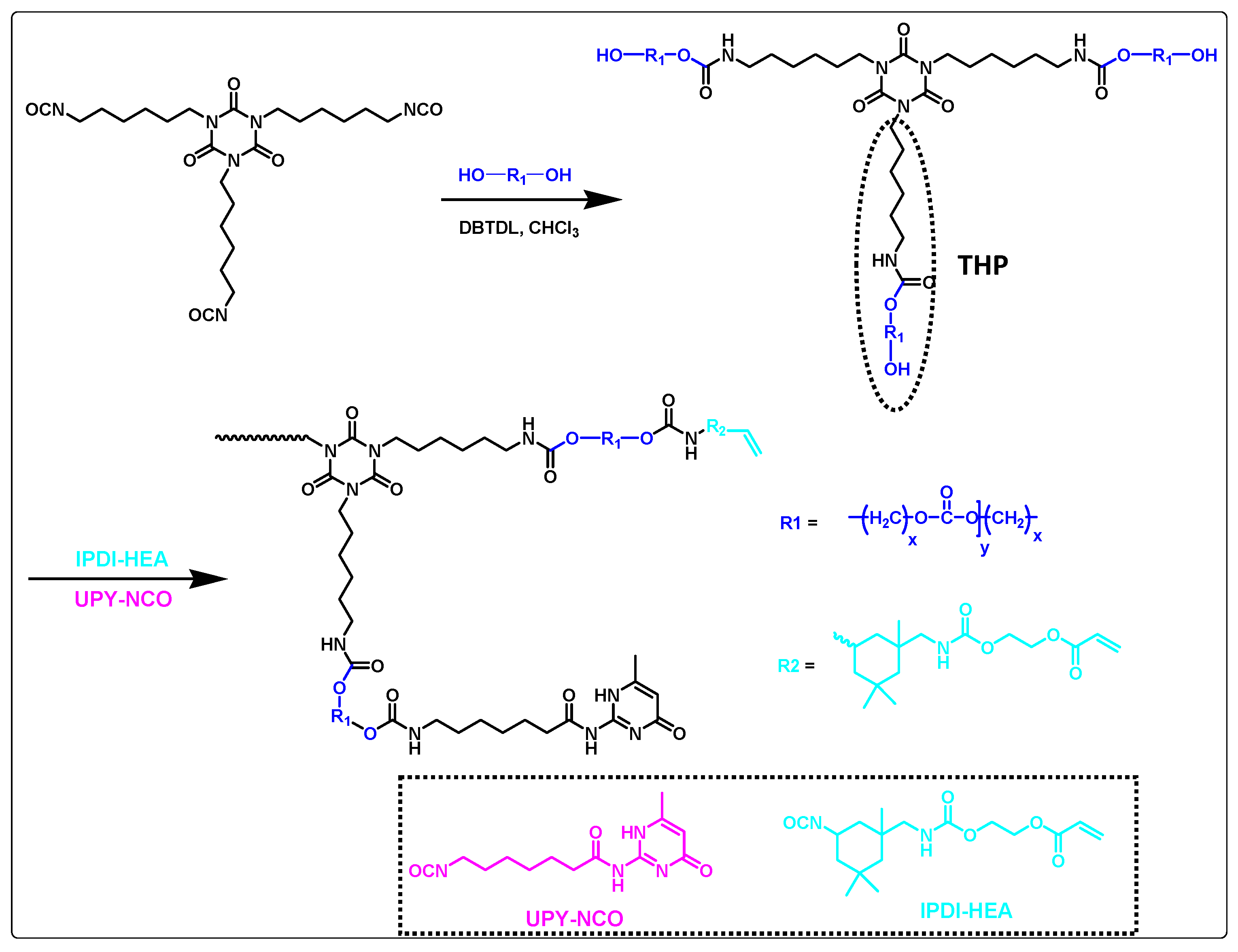
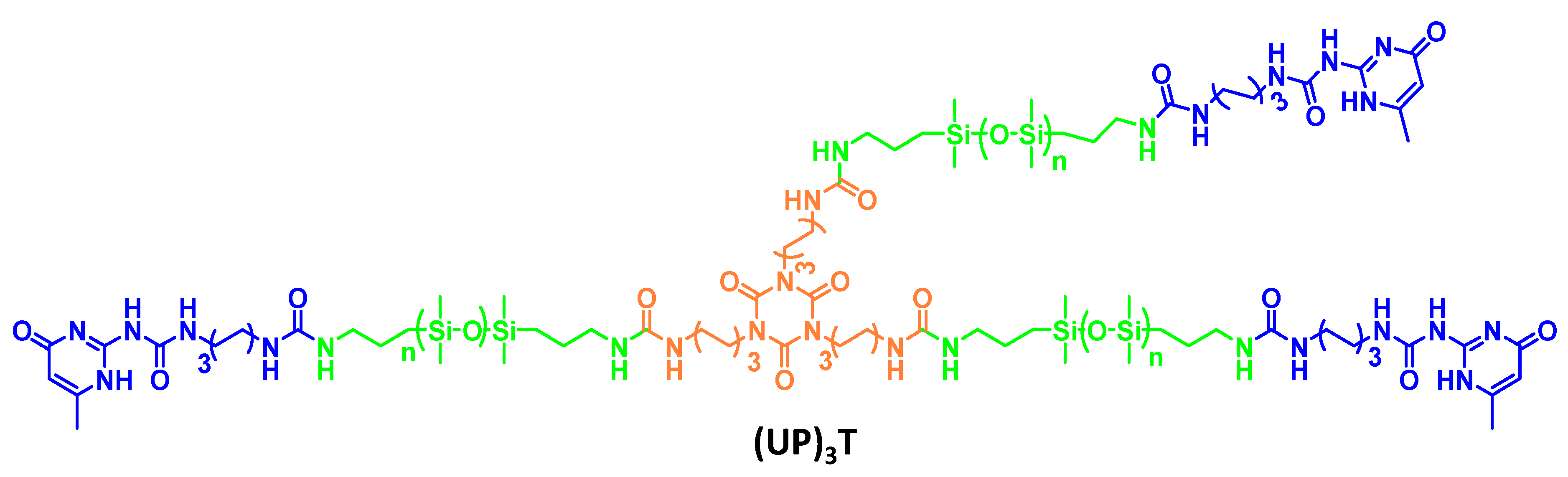
3.4. Ureidopyrimidone (UPy) Based Self-Healing Coating
3.5. Self-Healing Transparent Coating
4. Self-Healing via Carboxylated-Bonded Supramolecular Interactions
5. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Odland, G.; Ross, R. Human wound repair. I. Epidermal regeneration. J. Cell Biol. 1968, 39, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Herbst, F.; Döhler, D.; Mihael, P.; Binder, W.H. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, G.M.L.; Peeters, J.W.; Söntjens, S.H.M.; Janssen, H.M.; Bosman, A.W. Self-Healing Supramolecular Polymers In Action. Macromol. Chem. Phys. 2012, 213, 234–242. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing of polymers via supramolecular chemistry. Adv. Mater. Interfaces 2018, 1800384. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, X.; Sun, S.; Yu, C.; Xia, H. Preparation, characterization and properties of intrinsic self-healing elastomers. J. Mater. Chem. B 2019, 7, 4876–4926. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Stimuli responsive self-healing polymers: Gels, elastomers and membranes. Polym. Chem. 2017, 8, 6464. [Google Scholar] [CrossRef] [Green Version]
- Willocq, B.; Odent, J.; Dubois, P.; Raquez, J.-M. Advances in intrinsic self-healing polyurethanes and related composites. RSC Adv. 2020, 10, 13766. [Google Scholar] [CrossRef]
- Menon, A.V.; Madras, G.; Bose, S. The journey of self-healing and shape memory polyurethanes from bench to translational research. Polym. Chem. 2019, 10, 4370–4388. [Google Scholar] [CrossRef]
- Shchukin, D.G. Container-based multifunctional self-healing polymer coatings. Polym. Chem. 2013, 4, 4871. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Stephenson, L.D.; Murray, J.N. Self-healing coatings for steel. Prog. Org. Coat. 2006, 55, 244–253. [Google Scholar] [CrossRef]
- Samadzadeha, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; van der Zwaag, S. A critical appraisal of the potential of self healing polymeric coatings. Prog. Org. Coat. 2011, 72, 211–221. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown., E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Dry, C. Procedures developed for self-repair of polymer matrix composite materials. Compos. Struct. 1996, 35, 263–269. [Google Scholar] [CrossRef]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of Isocyanates for Self-Healing Polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Syrett, J.A.; Becer, C.R.; Haddleton, D.M. Self-healing and self-mendable polymers. Polym. Chem. 2010, 1, 978. [Google Scholar] [CrossRef]
- Young, S.; Arunbabu, D.; Noh, S.M.; Song, Y.K.; Oh, J.K. Recent strategies to develop self-healable crosslinked polymeric networks. Chem. Commun. 2015, 51, 13058–13070. [Google Scholar]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Coumarin imparts repeated photochemical remendability to polyurethane. J. Mater. Chem. 2011, 21, 18373–18380. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D. A Novel Self-Healing Polyurethane Based on Disulfide Bonds. Macromol. Chem. Phys. 2016, 217, 1191–1196. [Google Scholar] [CrossRef]
- Fan, W.; Jin, Y.; Huang, Y.; Pan, J.; Du, W.; Pu, Z. Room-temperature self-healing and reprocessing of Diselenide-containing waterborne polyurethanes under visible light. J. Appl. Polym. Sci. 2019, 136, 47071. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Ostsuka, H.; Takahara, A.; Matyjaszewski, K. Self-Healing of Covalently Cross-Linked Polymers by Reshuffling Thiuram Disulfide Moieties in Air under Visible Light. Adv. Mater. 2012. [CrossRef] [PubMed]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as Reversible Covalent Crosslinkers for Self-Healing Polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Dahlke, J.; Zechel, S.; Hager, M.D.; Schubert, U.S. How to Design a Self-Healing Polymer: General Concepts of Dynamic Covalent Bonds and Their Application for Intrinsic Healable Materials. Adv. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Bapat, A.P.; Sumerlin, B.S. Room-Temperature Self-Healing Polymers Based on Dynamic-Covalent Boronic Esters. Macromolecules 2015, 48, 2098–2106. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hill, M.R.; Sumerlin, B.S. Self-healing hydrogels containing reversible oxime crosslinks. Soft Matter 2015, 11, 6152–6161. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Tao, L.; Hsieh, F.-Y.; Wei, Y.; Chiu, I.-M.; Hsu, S.-H. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, 1706846. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gao, Y.; Jiang, S.; Sun, F. Photocured Materials with Self-Healing Function through Ionic Interactions for Flexible Electronics. ACS Appl. Mater. Interfaces 2018, 10, 26694–26704. [Google Scholar] [CrossRef]
- Li, C.-H.; Zuo, J.-L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π−π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host-guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Siva Prasanna Sanka, R.V.; Krishnakumar, B.; Leterrier, Y.; Pandey, S.; Rana, S.; Michaud, V. Soft Self-Healing Nanocomposites. Front. Mater. 2019, 6, 137. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Hillewaere, X.K.D.; Du Prez, F.E. Fifteen chemistries for autonomous external self-healing polymers and composites. Prog. Polym. Sci. 2015, 49–50, 121–153. [Google Scholar] [CrossRef]
- An, S.; Lee, M.W.; Yarin, A.L.; Yoon, S.S. A review on corrosion-protective extrinsic self-healing: Comparison of microcapsule-based systems and those based on core-shell vascular networks. Chem. Eng. J. 2018, 344, 206–220. [Google Scholar] [CrossRef]
- Golkaram, M.; Fodor, C.; van Ruymbeke, E.; Loos, K. Linear Viscoelasticity of Weakly Hydrogen-Bonded Polymers near and below the Sol−Gel Transition. Macromolecules 2018, 51, 4910–4916. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977. [Google Scholar] [CrossRef] [PubMed]
- Montarnal, D.; Tournilhac, F.; Hidalgo, M.; Couturier, J.-L.; Leibler, L. Versatile One-Pot Synthesis of Supramolecular Plastics and Self-Healing Rubbers. J. Am. Chem. Soc. 2009, 131, 7966. [Google Scholar] [CrossRef]
- Hart, L.R.; Harries, J.L.; Greenland, B.W.; Colquhoun, H.M.; Hayes, W. Healable supramolecular polymers. Polym. Chem. 2013, 4, 4860–4870. [Google Scholar] [CrossRef]
- Montarnal, D.; Tournilhac, F.; Hidalgo, M.; Leibler, L. Epoxy-based networks combining chemical and supramolecular hydrogen-bonding crosslinks. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1133–1141. [Google Scholar] [CrossRef]
- Wietor, J.-L.; Dimopoulos, A.; Govaert, L.E.; van Benthem, R.A.T.M.; de With, G.; Sijbesma, R.P. Preemptive Healing through Supramolecular Cross-Links. Macromolecules 2009, 42, 6640–6646. [Google Scholar] [CrossRef] [Green Version]
- Sordo, F.; Mougnier, S.-J.; Loureiro, N.; Tournilhac, F.; Michaud, V. Design of Self-Healing Supramolecular Rubbers with a Tunable Number of Chemical Cross-Links. Macromolecules 2015, 48, 4394–4402. [Google Scholar] [CrossRef]
- Wittmer, A.; Brinkmann, A.; Stenzel, V.; Hartwig, A.; Koschek, K. Moisture-mediated intrinsic self-healing of modified polyurethane urea polymers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 537–548. [Google Scholar] [CrossRef]
- Wittmer, A.; Brinkmann, A.; Stenzel, V.; Koschek, K. Stimuli-responsive polyurethane-urea polymer for protective coatings and dampening material. J. Coat. Technol. Res. 2019, 16, 189–197. [Google Scholar] [CrossRef]
- Willocq, B.; Khelifa, F.; Odent, J.; Lemaur, V.; Yang, Y.; Leclere, P.; Cornil, J.; Dubois, P.; Urban, M.W.; Raquez, J.-M. Mechanistic Insights on Spontaneous Moisture-Driven Healing of Urea-Based Polyurethanes. ACS Appl. Mater. Interfaces 2019, 11, 46176–46182. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qiu, W.-Z.; Wu, Z.L.; Ren, P.-F.; Zheng, Q.; Xu, Z.-K. Water-Triggered Self-Healing Coatings of Hydrogen-Bonded Complexes for High Binding Affinity and Antioxidative Property. Adv. Mater. Interfaces 2016. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 125, 10966. [Google Scholar] [CrossRef] [Green Version]
- Bahorun, T.; Neergheen-Bhujun, V.; Toolsee, N.A.; Somanah, J.; Luximon-Ramma, A.; Aruoma, O.I. Tea in Health and Disease Prevention; Academic Press: New York, NY, USA, 2013; p. 361. [Google Scholar]
- Zhang, X.; Ren, P.-F.; Yang, H.-C.; Wan, L.-S.; Xu, Z.-K. Co-deposition of tannic acid and diethlyenetriamine for surface hydrophilization of hydrophobic polymer membranes. Appl. Surf. Sci. 2016, 360, 291. [Google Scholar] [CrossRef]
- Ünal, H. Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2018; pp. 301–319. [Google Scholar]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, J.; Luo, J.; Liu, X. Synthesis and application of novel UV-curable hyperbranched methacrylates from renewable natural tannic acid. Prog. Org. Coat. 2014, 77, 30–37. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Patani, R.; Wu, J.F.; Fernando, S.; Jogodzinski, K.; Webster, D.C. Soy-based UV-curable thiol–ene coatings. J. Coat. Technol. Res. 2010, 7, 603–613. [Google Scholar] [CrossRef]
- Gao, Q.; Li, H.; Zeng, X. Preparation and characterization of UV-curable hyperbranched polyurethane acrylate. J. Coat. Technol. Res. 2011, 8, 61–66. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-based, high biorenewable content UV curable coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- Tasic, S.; Bozic, B.; Dunjic, B. Synthesis of new hyperbranched urethane-acrylates and their evaluation in UV-curable coatings. Prog. Org. Coat. 2004, 51, 321–328. [Google Scholar] [CrossRef]
- Arimitsu, K.; Kobayashi, H.; Furutani, M.; Gunji, T.; Abe, Y. Base-amplifying silicone resins generating aliphatic secondary amines autocatalytically: Synthesis, characterization and application to positive-working photoresists. Polym. Chem. 2014, 5, 6671–6677. [Google Scholar] [CrossRef]
- Giardi, R.; Porro, S.; Chiolerio, A.; Celasco, E.; Sangermano, M. Inkjet printed acrylic formulations based on UV-reduced graphene oxide nanocomposites. J. Mater. Sci. 2013, 48, 1249–1255. [Google Scholar] [CrossRef]
- Sijbesma, R.; Beijer, F.; Brunsveld, L.; Folmer, B.J.; Hirschberg, J.; Lange, R.; Lowe, J.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Beijer, F.H.; Sijbesma, R.P.; Kooijman, H.; Spek, A.L.; Meijer, E.W. Strong Dimerization of Ureidopyrimidones via Quadruple Hydrogen Bonding. J. Am. Chem. Soc. 1998, 120, 6761–6769. [Google Scholar] [CrossRef]
- Wei, M.; Zhan, M.; Yu, D.; Xie, H.; He, M.; Yang, K.; Wang, Y. Novel Poly(tetramethylene ether)glycol and Poly(ε-caprolactone) Based Dynamic Network via Quadruple Hydrogen Bonding with Triple-Shape Effect and Self-Healing Capacity. ACS Appl. Mater. Interfaces 2015, 7, 2585–2596. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Z.; Shou, Y.; Zhang, K.; Li, G.; Xia, P.; Yan, S.; Yin, J. Stack-Based Hydrogels with Mechanical Enhancement, High Stability, Self-Healing Property, and Thermoplasticity from Poly(l-glutamic acid) and Ureido-Pyrimidinone. ACS Biomater. Sci. Eng. 2020, 6, 1715–1726. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, D. Self-healing supramolecular waterborne polyurethane dispersions with quadruple hydrogen bonds in main chain. J. Appl. Polym. Sci. 2020. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards dynamic, but supertough healable polymers via biomimetic hierarchical hydrogen bonding interaction. Angew. Chem. Int. Ed. 2018. [Google Scholar] [CrossRef]
- Liu, R.; Yang, X.; Yuan, Y.; Liu, J.; Liu, X. Synthesis and properties of UV-curable self-healing oligomer. Prog. Org. Coat. 2016, 101, 122–129. [Google Scholar] [CrossRef]
- Gao, F.; Cao, J.; Wang, Q.; Liu, R.; Zhang, S.; Liu, J.; Liu, X. Properties of UV-cured self-healing coatings prepared with PCDL-based polyurethane containing multiple H-bonds. Prog. Org. Coat. 2017, 113, 160–167. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D. Synthesis and properties of self-healing waterborne polyurethanes containing disulfide bonds in the main chain. J. Mater. Sci. 2017, 52, 197–207. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Zhou, Z.; Liu, R.; Yuan, Y.; Liu, X. Stiff Self-Healing Coating Based on UV-Curable Polyurethane with a “Hard Core, Flexible Arm” Structure. ACS Omega 2018, 3, 11128–11135. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, Q.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. Polydimethylsiloxane Coating for a Palladium/MOF Composite: Highly Improved Catalytic Performance by Surface Hydrophobization. Angew. Chem. Int. Ed. 2016, 55, 7379–7383. [Google Scholar] [CrossRef]
- Lu, N.; Kim, D.-H. Flexible and Stretchable Electronics Paving the Way for Soft Robotics. Soft Robot 2014, 1, 53–62. [Google Scholar] [CrossRef]
- Jeong, S.H.; Zhang, S.; Hjort, K.; Hilborn, J.; Wu, Z. PDMS-Based Elastomer Tuned Soft, Stretchable, and Sticky for Epidermal Electronics. Adv. Mater. 2016, 28, 5830–5836. [Google Scholar] [CrossRef]
- Gong, X.; Wen, W. Polydimethylsiloxane-based conducting composites and their applications in microfluidic chip fabrication. Biomicrofluidics 2009, 3, 012007. [Google Scholar] [CrossRef] [Green Version]
- Gohil, S.V.; Suhail, S.; Rose, J.; Vella, T.; Nair, L.S. Polymers and composites for orthopedic applications. In Materials for Bone Disorders; Academic Press: Cambridge, MA, USA, 2017; pp. 349–403. [Google Scholar]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent progress in fabrication and application of polydimethylsiloxane sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Liu, M.; Liu, P.; Lu, G.; Xu, Z.; Yao, X. Multiphase-Assembly of Siloxane Oligomers with Improved Mechanical Strength and Water-Enhanced Healing. Angew. Chem. Int. Ed. 2018. [CrossRef]
- Hentschel, J.; Kushner, A.M.; Ziller, J.; Guan, Z. Self-healing supramolecular block copolymers. Angew. Chem. Int. Ed. 2012, 51, 10561–10565. [Google Scholar] [CrossRef] [PubMed]
- Balkenende, D.W.R.; Monnier, C.A.; Fiore, G.L.; Weder, C. Optically responsive supramolecular polymer glasses. Nat. Commun. 2016, 7, 10995. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, Z.; Liu, P.; Wang, Z.; Yao, H.; Yao, X. Supramolecular silicone coating capable of strong substrate bonding, readily damage healing, and easy oil sliding. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Meneghetti, M. Advances in self-healing optical materials. J. Mater. Chem. 2012, 22, 24501. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Xu, L.; Zhang, X.; Zhang, A.; Xu, Y. A highly stretchable, transparent, notch-insensitive self-healing elastomer for coating. J. Mater. Chem. C 2020, 8, 2043–2053. [Google Scholar] [CrossRef]
- Jucius, D.; Lazauskas, A.; Gudaitis, R. Multiple Hydrogen-Bonding Assisted Scratch–Healing of Transparent Coatings. Coatings 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Macleod, D.M. Wire-Wound Rod Coating. In Coatings Technology: Fundamentals, Testing, and Processing Techniques; Tracton, A.A., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Chapter 19. [Google Scholar]
- Wang, X.; Wang, Y.; Bi, S.; Wang, Y.; Chen, X.; Qiu, L.; Sun, J. Optically Transparent Antibacterial Films Capable of Healing Multiple Scratches. Adv. Funct. Mater. 2013. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, S.; Guo, R.; Sun, J. Healable and Optically Transparent Polymeric Films Capable of Being Erased on Demand. ACS Appl. Mater. Interfaces 2015, 7, 13597–13603. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Yoo, D.; Lee, J.K.; Rubner, M.F. Solid-State Light-Emitting Devices Based on the Tris-Chelated Ruthenium(II) Complex: 3. High Efficiency Devices via a Layer-by-Layer Molecular-Level Blending Approach. J. Am. Chem. Soc. 1999, 121, 4883–4891. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hong, J.; Kavian, R.; Lee, S.W.; Hyder, M.N.; Shao-Horn, Y.; Hammond, P.T. Rapid fabrication of thick spray-layer-by-layer carbon nanotube electrodes for high power and energy devices. Energy Environ. Sci. 2013, 6, 888–897. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fang, X.; Wang, Y.; Ma, B.; Sun, J. Highly Transparent and Water-Enabled Healable Antifogging and Frost-Resisting Films Based on Poly(vinyl alcohol)–Nafion Complexes. Chem. Mater. 2016, 28, 6975–6984. [Google Scholar] [CrossRef]
- Reisch, A.; Roger, E.; Phoeung, T.; Antheaume, C.; Orthlieb, C.; Boulmedais, F.; Lavalle, P.; Schlenoff, J.B.; Frisch, B.; Schaaf, P. On the Benefits of Rubbing Salt in the Cut: Self-Healing of Saloplastic PAA/PAH Compact Polyelectrolyte Complexes. Adv. Mater. 2014, 26, 2547–2551. [Google Scholar] [CrossRef] [Green Version]
- Yilgor, I.; Eynur, T.; Yilgor, E.; Wilkes, G.L. Contribution of soft segment entanglement on the tensile properties of silicone–urea copolymers with low hard segment contents. Polymer 2009, 50, 4432–4437. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; AIP Press: New York, NY, USA, 2007. [Google Scholar]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, S.; Li, T.; Song, Y.; Li, Z.; Zhang, W.; Sun, J. Transparent, Healable Elastomers with High Mechanical Strength and Elasticity Derived from Hydrogen-Bonded Polymer Complexes. ACS Appl. Mater. Interfaces 2017, 9, 29120–29129. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Mather, P.T. Shape Memory Assisted Self-Healing Coating. ACS Macro Lett. 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Lazauskas, A.; Jucius, D.; Baltrusaitis, V.; Gudaitis, R.; Prosycevas, I.; Abakeviciene, B.; Guobiene, A.; Andrulevicius, M.; Grigaliunas, V. Shape-Memory Assisted Scratch-Healing of Transparent Thiol-Ene Coatings. Materials 2019, 12, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.-Y.; Wang, H.-Q.; Lai, J.-C.; Li, C.-H. A Self-Healing and Shape Memory Polymer that Functions at Body Temperature. Molecules 2019, 24, 3224. [Google Scholar] [CrossRef] [Green Version]









| Polymer | Tg °C | Healing Condition | Healing Efficiency (%) | Appearance | Ref. |
|---|---|---|---|---|---|
| Polyurethane Urea | 5–25 | 24 h, RH 50%, RT | NA | Transparent | [52] |
| Polyurethane Urea | −1 and 69 | 6 days, RH 90%, RT, and 24 h, 80 °C | NA | Transparent | [53] |
| Urea based polyurethane | 12 | 4 h, RH 97%, RT | NA | Transparent | [54] |
| Tannic acid (TA)-polyethylene glycol (PEG) | NA | 5 min, water, RT, and 30 min, RH 100%, RT | NA | Transparent | [55] |
| Polystyrene and polyacrylate amide-based brush polymer | 2–5 | 24 h, Ambient conditions | 92 | Non-transparent | [61] |
| UV curved polyurethane | 15–25 | Healed using heat gun for 1 min with temperature set of 170 °C at a 10 cm distance to surface | 96 | NA | [76] |
| UV curved polyurethane | NA | 1–2 min, 70 °C | 98 | NA | [77] |
| UV-curable polyurethane | 15.5 | 5–10 min, 100 °C | NA | NA | [89] |
| Silicone based | 34 | 5 min, water, 70 °C 12 h, RH 90%, 70 °C | >90 | Transparent | [85] |
| Polyurea elastomer | −57 | 6 h, 25 °C | >99 | Transparent | [90] |
| poly(vinyl alcohol) and poly(acrylic acid) complex | NA | 24 h, RH 40%, RT and 30 min, RH 90%, RT | >95 | Transparent | [91] |
| Polyethylenimine /poly(acrylic acid) complex | NA | 30 min, water, RT | NA | Transparent | [93] |
| poly(acrylic acid) and poly(ethylene oxide) solution complex | NA | 30 min, pH 2.5, RT | NA | Transparent | [94] |
| poly(acrylic acid) and poly(ethylene oxide) bulk complex | 20.5 | 24 h, RH 90%, RT | >95 | Transparent | [95] |
| (PDMS-COOH) and (PEGDGE) complex | 0.5 | 6 h, RT | >97 | Transparent | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadwal, I. A Brief Overview on Preparation of Self-Healing Polymers and Coatings via Hydrogen Bonding Interactions. Macromol 2021, 1, 18-36. https://doi.org/10.3390/macromol1010003
Gadwal I. A Brief Overview on Preparation of Self-Healing Polymers and Coatings via Hydrogen Bonding Interactions. Macromol. 2021; 1(1):18-36. https://doi.org/10.3390/macromol1010003
Chicago/Turabian StyleGadwal, Ikhlas. 2021. "A Brief Overview on Preparation of Self-Healing Polymers and Coatings via Hydrogen Bonding Interactions" Macromol 1, no. 1: 18-36. https://doi.org/10.3390/macromol1010003




