Biosensor for Detecting Fetal Growth Restriction in a Low-Resource Setting
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pels, A.; Beune, I.M.; Van Wassenaer-Leemhuis, A.G.; Limpens, J.; Ganzevoort, W. Early-onset fetal growth restriction: A systematic review on mortality and morbidity. Acta Obstet. Gynecol. Scand. 2020, 99, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Gardosi, J.; Madurasinghe, V.; Williams, M.; Malik, A.; Francis, A. Maternal and fetal risk factors for stillbirth: Population-based study. BMJ 2013, 68, 329–331. [Google Scholar] [CrossRef]
- Crispi, F.; Miranda, J.; Gratacós, E. Longterm cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018, 218, 869–879. [Google Scholar] [CrossRef]
- Kingdom, J.C.; Audette, M.C.; Hobson, S.R.; Windrim, R.C.; Morgen, E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S803–S817. [Google Scholar] [CrossRef]
- Ghamari, M.; Janko, B.; Sherratt, R.S.; Harwin, W.; Piechocki, R.J.; Soltanpur, C. A Survey on Wireless Body Area Networks for eHealthcare Systems in Residential Environments. Sensors 2016, 16, 831. [Google Scholar] [CrossRef]
- Riknagel, D.; Farlie, R.; Hedegaard, M.; Humaidan, P.; Struijk, J.J. Association between maternal vascular murmur and the small-for-gestational-age fetus with abnormal umbilical artery Doppler flow. Int. J. Gynecol. Obstet. 2017, 139, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Riknagel, D.; Zimmermann, H.; Farlie, R.; Hammershøi, D.; E Schmidt, S.; Hedegaard, M.; Humaidan, P.; Struijk, J.J. Separation and characterization of maternal cardiac and vascular sounds in the third trimester of pregnancy. Int. J. Gynecol. Obstet. 2017, 137, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Riknagel, D.; Dinesen, B.; Zimmermann, H.; Farlie, R.; Schmidt, S.E.; Toft, E.; Struijk, J.J. Digital auscultation of the uterine artery: A measure of uteroplacental perfusion. Physiol. Meas. 2016, 37, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.E.; Holst-Hansen, C.; Hansen, J.; Toft, E.; Struijk, J.J. Acoustic Features for the Identification of Coronary Artery Disease. IEEE Trans. Biomed. Eng. 2015, 62, 2611–2619. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology: A Cellular and Molecular Approach, 2nd ed.; Saunders, P.A., Ed.; Elsevier: Philadelphia, PA, USA, 2012; Volume xiii, p. 1337. [Google Scholar]
- Roth, C.J.; Haeussner, E.; Ruebelmann, T.; Koch, F.V.; Schmitz, C.; Frank, H.-G.; Wall, W.A. Dynamic modeling of uteroplacental blood flow in IUGR indicates vortices and elevated pressure in the intervillous space—A pilot study. Sci. Rep. 2017, 7, 40771. [Google Scholar] [CrossRef]
- Burton, G.; Woods, A.; Jauniaux, E.; Kingdom, J. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Bulfamante, G.P.; Morabito, A.; Marconi, A.M. Abnormal spiral artery remodelling in the decidual segment during pregnancy: From histology to clinical correlation. J. Clin. Pathol. 2011, 64, 1064–1068. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Hjort, L.; Møller, S.L.; Minja, D.; Msemo, O.; Nielsen, B.B.; Christensen, D.L.; Theander, T.; Nielsen, K.; Larsen, L.G.; Grunnet, L.G.; et al. FOETAL for NCD—FOetal Exposure and Epidemiological Transitions: The role of Anaemia in early Life for Non-Communicable Diseases in later life: A prospective preconception study in rural Tanzania. BMJ Open 2019, 9, e024861. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Kennedy, S.H.; Salomon, L.J.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Lambert, A.; Carvalho, M.; Jaffer, Y.A.; Bertino, E.; et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown–rump length in the first trimester of pregnancy. Ultrasound Obstet. Gynecol. 2014, 44, 641–648. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Kemp, B.; Stones, W.; Ohuma, E.O.; Kennedy, S.; Purwar, M.; Salomon, L.J.; Altman, D.G.; Noble, J.A.; Bertino, E.; et al. Ultrasound-based gestational-age estimation in late pregnancy. Ultrasound Obstet. Gynecol. 2016, 48, 719–726. [Google Scholar] [CrossRef]
- The International Fetal and Newborn Growth Consortium. INTERGROWTH 21st Ultrasound Operations Manual; University of Oxford: Oxford, UK, 2009. [Google Scholar]
- Nicolaides, K.H.; Rizzo, G.; Hecher, K.X. Doppler in Obstetrics—Diploma in Fetal Medicine and ISUOG Educational Series; The Fetal Medicine Foundation: London, UK, 2002. [Google Scholar]
- Lefebvre, J.; Demers, S.; Bujold, E.; Nicolaides, K.H.; Girard, M.; Brassard, N.; Audibert, F. Comparison of two different sites of measurement for transabdominal uterine artery Doppler velocimetry at 11-13 weeks. Ultrasound Obstet. Gynecol. 2012, 40, 288–292. [Google Scholar] [CrossRef]
- Gómez, O.; Figueras, F.; Fernández, S.; Bennasar, M.; Martínez, J.M.; Puerto, B.; Gratacós, E. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008, 32, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cordero, M.; Lees, C.; Missfelder-Lobos, H.; Seed, P.; Harris, C. Fetal arterial and venous Doppler pulsatility index and time averaged velocity ranges. Prenat. Diagn. 2007, 27, 1251–1257. [Google Scholar] [CrossRef]
- Schmiegelow, C.; Scheike, T.H.; Oesterholt, M.; Minja, D.; Pehrson, C.; Magistrado, P.; Lemnge, M.; Rasch, V.; Lusingu, J.; Theander, T.G.; et al. Development of a Fetal Weight Chart Using Serial Trans-Abdominal Ultrasound in an East African Population: A Longitudinal Observational Study. PLoS ONE 2012, 7, e44773. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Impact Model Group (Anemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Andreasen, L.A.; Tabor, A.; Nørgaard, L.N.; Rode, L.; Gerds, T.A.; Tolsgaard, M.G. Detection of growth-restricted fetuses during pregnancy is associated with fewer intrauterine deaths but increased adverse childhood outcomes: An observational study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Caradeux, J.; Martinez-Portilla, R.J.; Peguero, A.; Sotiriadis, A.; Figueras, F. Diagnostic performance of third-trimester ultrasound for the prediction of late-onset fetal growth restriction: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 449–459.e19. [Google Scholar] [CrossRef]
- Riknagel, D.; Al Humaidan, P.H.; Farlie, R.; Zimmermann, H.; Ramsing, M.; Struijk, J.J. Acoustic biomarker of placental pathophysiology and adverse fetal outcome. Placenta 2015, 36, A6. [Google Scholar] [CrossRef]

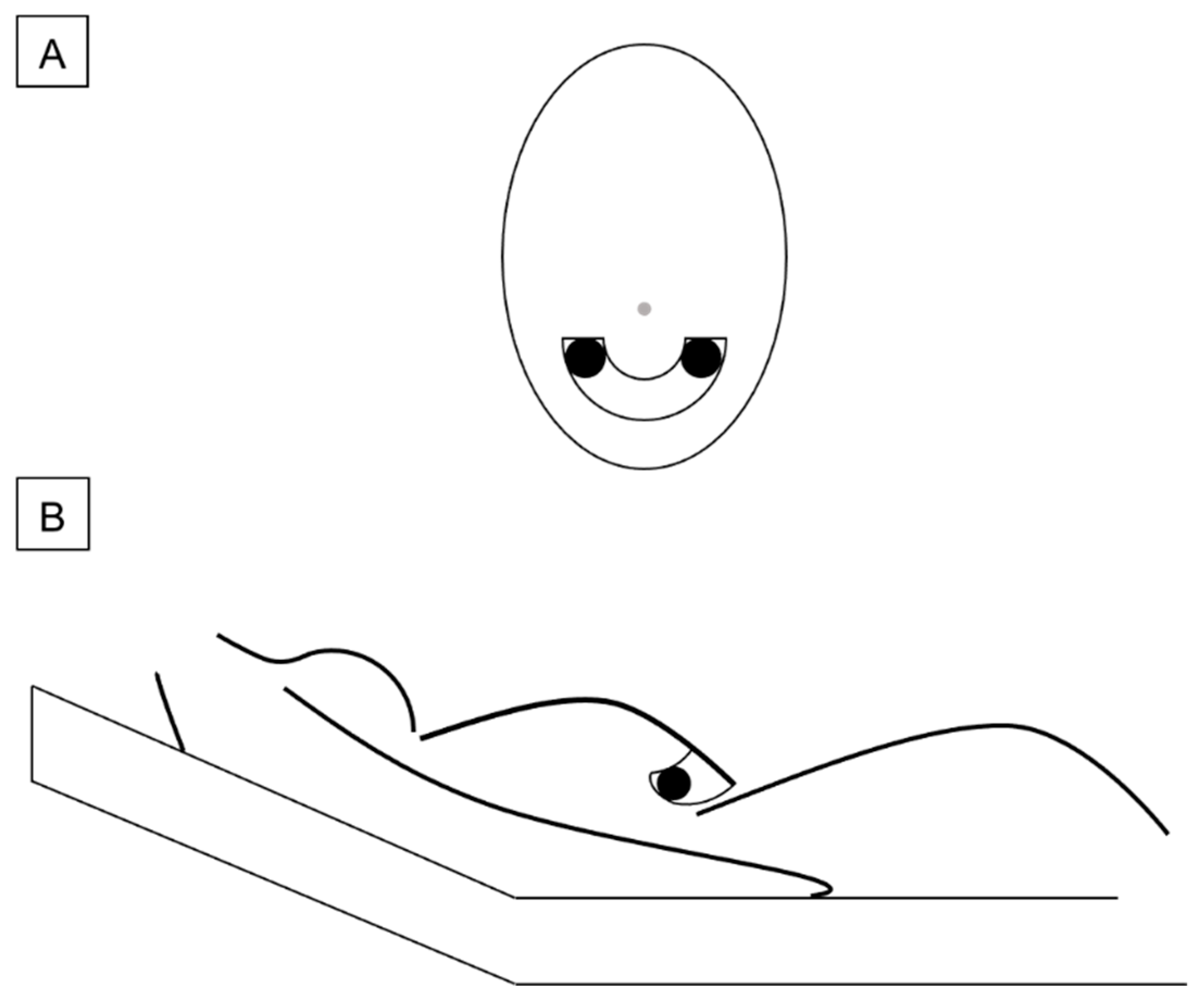
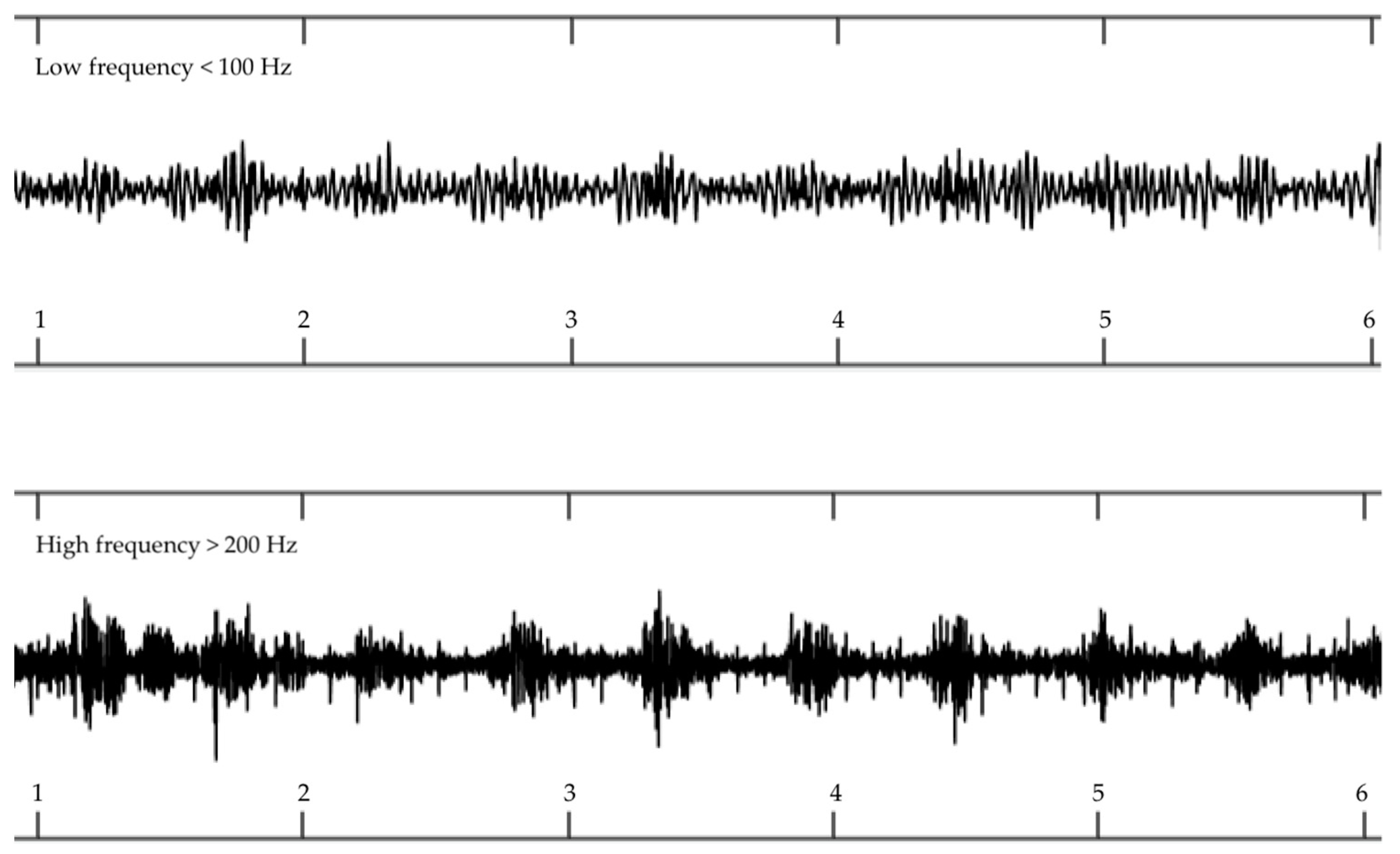
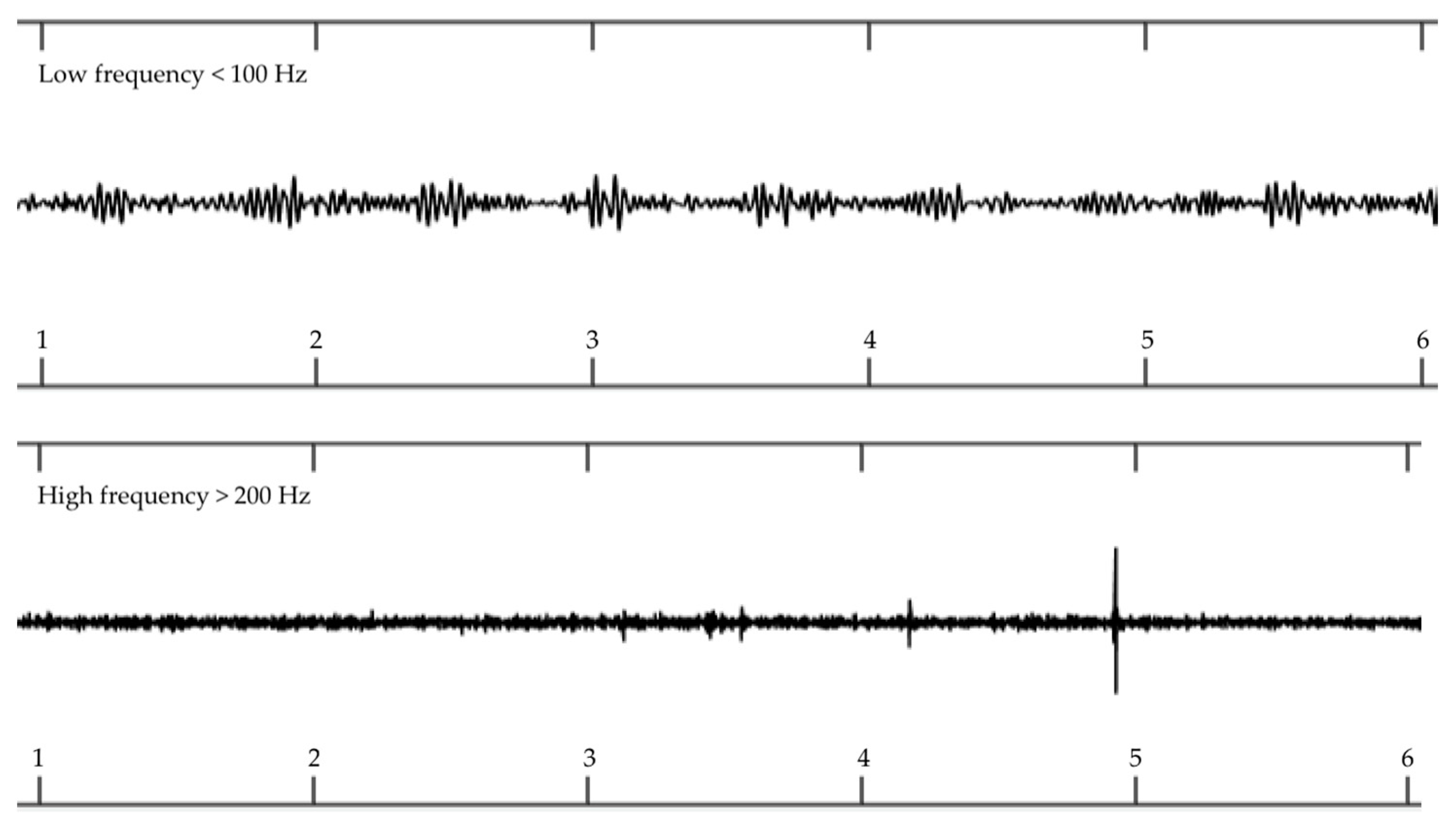
| Quality Index | Cardiac Valve Frequency 1 | Arterial Turbulence Frequency 1 |
|---|---|---|
| 1 | 390 | 294 |
| 2 | 824 | 1022 |
| 3 | 472 | 388 |
| 4 | 192 | 174 |
| Total (n) | 1878 | 1878 |
| Variable | Mean | Min–Max | SD |
|---|---|---|---|
| Pregnancy length, d | 280 | 259–301 | 8 |
| Birth weight, g | 3008 | 1565–4025 | 410 |
| Placenta weight, g | 440 | 265–867 | 107 |
| Systolic BP | 107 | 78–151 | 11 |
| Diastolic BP | 68 | 43–110 | 10 |
| Variable | MAT+ (n = 5) | MAT− (n = 113) | 95% CI for Difference of Means | p-Value |
|---|---|---|---|---|
| Pregnancy length, d | 280 ± 12 | 280 ± 8 | −7.5 to 6.9 | 0.9 1 |
| BW, g | 2689 ± 642 | 3022 ± 395 | −34.7 to 70.7 | 0.08 1 |
| Placenta weight, g | 450 ± 53 | 440 ± 108 | −118.3 to 97.4 | 0.8 1 |
| Systolic BP | 117 ± 1.3 | 106 ± 13 | −22.5 to 0.5 | 0.06 1 |
| Diastolic BP | 77 ± 6 | 69 ± 11 | −17.5 to 0.6 | 0.1 1 |
| Hemoglobin, g/dL | 11.7 ± 1.3 | 10.8 ± 1.4 | −2.2 to 0.4 | 0.2 1 |
| EFW GA 26–28 | 981 ± 111 | 926 ± 140 | −256 to 146 | 0.6 1 |
| EFW GA 32–34 | 1629 ± 265 | 1844 ± 267 | −55 to 485 | 0.1 1 |
| EFW GA 37–39 | 2502 ± 166 | 2822 ± 317 | 3.8 to 637 | 0.04 1 |
| UA PI GA 26–28 | 0.95 ± 0.3 | 1.06 ± 0.2 | −0.1 to 0.3 | 0.4 1 |
| UA PI GA 32–34 | 0.89 ± 0.2 | 0.93 ± 0.2 | −0.2 to 0.2 | 0.7 1 |
| UA PI GA 37–39 | 0.99 ± 0.2 | 0.85 ± 0.4 | −0.3 to 0.02 | 0.9 2 |
| UtA PI GA 26–28 | 0.81 ± 0.2 | 0.83 ± 0.2 | −0.1 to 0.1 | 0.8 1 |
| UtA PI GA 32–34 | 0.71 ± 0.1 | 0.66 ± 0.2 | −0.1 to 0.3 | 0.4 1 |
| UtA PI GA 37–39 | 0.69 ± 0.1 | 0.68 ± 0.1 | −0.1 to 0.1 | 0.9 1 |
| Biosensor Test | Condition Positive | Condition Negative | PPV and NPV |
|---|---|---|---|
| Test outcome positive 1 | TP = 2 FP = 5 | FN = 3 TN = 108 | 2/(2 + 3) = 0.40 108/(108 + 5) = 0.96 |
| Sensitivity | 2(2 + 5) = 0.29 | ||
| Specificity | 108/(108 + 3) = 0.97 |
| Biosensor Test | Condition Positive | Condition Negative | PPV and NPV |
|---|---|---|---|
| Test outcome positive 1 | TP = 1 FP = 0 | FN = 4 TN = 113 | 1/(1 + 4) = 0.20 113/(113 + 0) = 1.00 |
| Sensitivity | 1(1 + 0) = 1.00 | ||
| Specificity | 113/(113 + 4) = 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobsen, A.; Schmiegelow, C.; Sørensen, B.; Msemo, O.A.; Nielsen, K.; Nielsen, B.B.; Møller, S.L.; Lusingu, J.P.A.; Minja, D.T.R.; Hedegaard, M.; et al. Biosensor for Detecting Fetal Growth Restriction in a Low-Resource Setting. Reprod. Med. 2021, 2, 57-67. https://doi.org/10.3390/reprodmed2010007
Jacobsen A, Schmiegelow C, Sørensen B, Msemo OA, Nielsen K, Nielsen BB, Møller SL, Lusingu JPA, Minja DTR, Hedegaard M, et al. Biosensor for Detecting Fetal Growth Restriction in a Low-Resource Setting. Reproductive Medicine. 2021; 2(1):57-67. https://doi.org/10.3390/reprodmed2010007
Chicago/Turabian StyleJacobsen, Anders, Christentze Schmiegelow, Bjarke Sørensen, Omari A. Msemo, Karsten Nielsen, Birgitte Bruun Nielsen, Sofie Lykke Møller, John P. A. Lusingu, Daniel T. R. Minja, Morten Hedegaard, and et al. 2021. "Biosensor for Detecting Fetal Growth Restriction in a Low-Resource Setting" Reproductive Medicine 2, no. 1: 57-67. https://doi.org/10.3390/reprodmed2010007
APA StyleJacobsen, A., Schmiegelow, C., Sørensen, B., Msemo, O. A., Nielsen, K., Nielsen, B. B., Møller, S. L., Lusingu, J. P. A., Minja, D. T. R., Hedegaard, M., & Riknagel, D. (2021). Biosensor for Detecting Fetal Growth Restriction in a Low-Resource Setting. Reproductive Medicine, 2(1), 57-67. https://doi.org/10.3390/reprodmed2010007







