Systematic Development, Validation and Optimization of a Human Embryo Culture System
Abstract
:1. Introduction
2. Materials and Methods
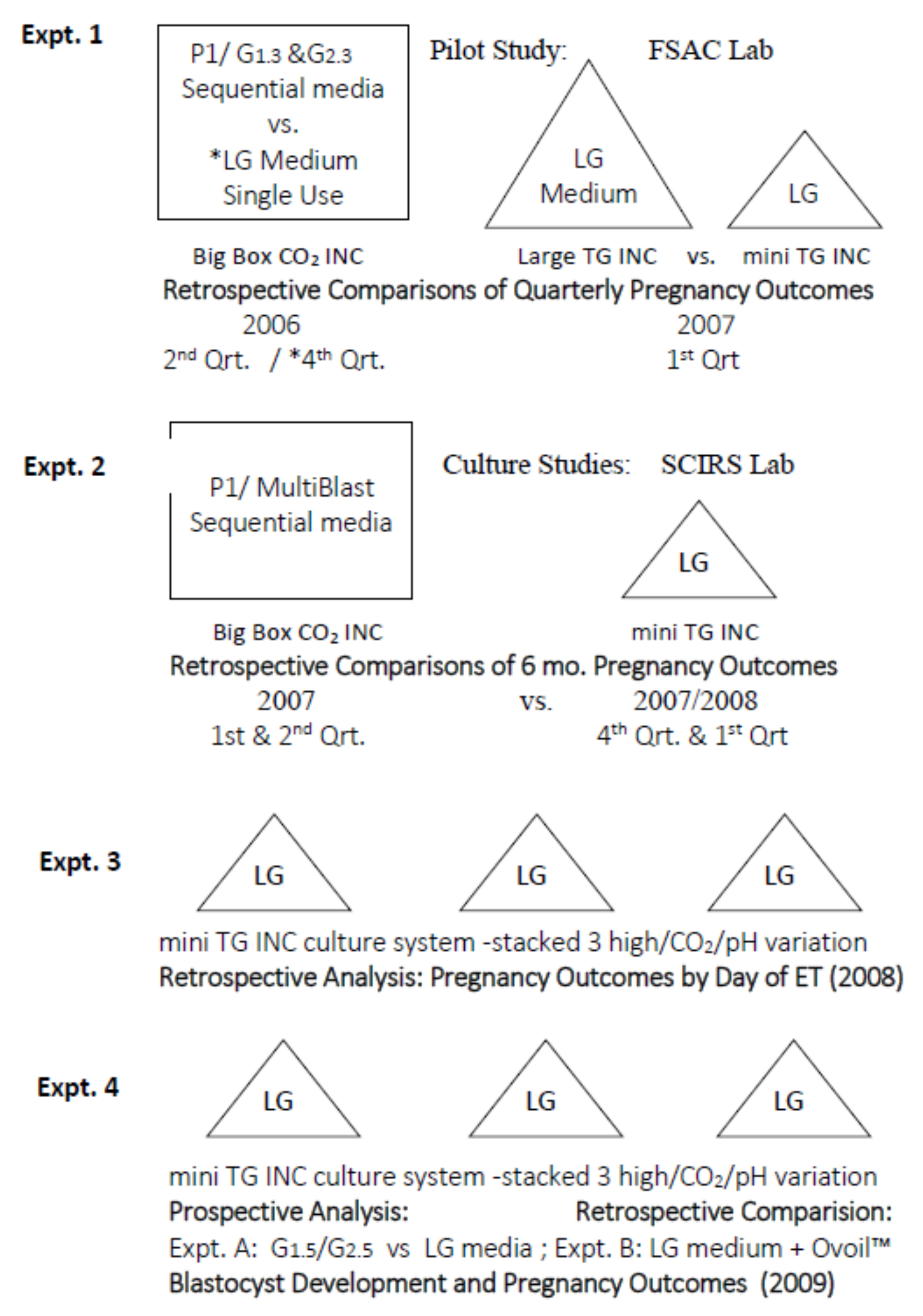
2.1. Experimental Design and Objectives
2.1.1. Informed Patient Consent
2.1.2. Phase 1. Development and Validation of Embryo Culture Systems
2.1.3. Phase 2. Verification of an Optimized Embryo Culture System
2.2. Statistical Analysis
2.3. General ART Applications
2.4. Incubator Conditions and Quality Control
2.5. Embryo Assessments and Procedural Manipulations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitten, W.K. Culture of tubal ova. Nature 1957, 179, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Brinster, R.L. Studies on the development of mouse embryos in vitro. II. The effects of energy source. J. Exp. Zool. 1965, 158, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Biggers, J.D.; Moore, B.D.; Whittingham, D.G. Development of mouse embryos in vivo after cultivation from two-cell ova to blastocysts in vitro. Nature 1965, 206, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, D.G. Culture of mouse ova. J. Reprod. Fertil. 1971, 14, 7–21. [Google Scholar]
- Biggers, J.D. Pioneering mammalian embryo culture. In The Mammalian Preimplantation Embryo Regulation of Growth and Differentiation in vitro; Bavister, B.D., Ed.; Plenum Press: New York, NY, USA, 2002; pp. 1–22. [Google Scholar]
- Summers, M.C.; Biggers, J.D. Chemically defined media and the culture of mammalian preimplantation embryos: Historical perspective and the current issues. Hum. Reprod. Update 2003, 9, 557–582. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, H.I.; Ali, J. Embryo culture media, culture techniques and embryo selection: A tribute to Wesley Kingston Whitten. J. Reprod. Stem Cell Biotechnol. 2010, 11, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Eagle, H. Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef]
- Ham, R.G. An improved nutrient solution for diploid Chinese hamster cells in a synthetic medium supplemented with purified protein fractions. Exp. Cell Res. 1963, 29, 515–526. [Google Scholar] [CrossRef]
- Whitten, W.K.; Biggers, J.D. Complete development in vitro of preimplantation stages of the mouse in a simple defined medium. J. Reprod. Fertil. 1968, 17, 399–401. [Google Scholar] [CrossRef] [Green Version]
- Leese, H.J. Metabolism control during preimplantation mammalian development. Hum. Reprod. Update 1995, 1, 63–72. [Google Scholar] [CrossRef]
- Leese, H.J. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. Bioessays 2002, 24, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.; Kerin, J.F.; Warnes, G.M. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil. Steril. 1985, 44, 493–498. [Google Scholar] [CrossRef]
- Menezo, Y.L.; Guenn, J.F.; Czyba, J.C. Improvement of human early embryo development in vitro by coculture on monolayers of vero cells. Biol. Reprod. 1990, 42, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongso, A.; Fong, C.Y.; Ng, S.C.; Ratnam, S. The search for improved in-vitro systems should not be ignored: Embryo co-culture may be one of them. Hum. Reprod. 1993, 8, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Lawitts, J.A.; Biggers, J.D. Optimization of mouse embryo culture media using simplex methods. J. Reprod. Fertil. 1991, 91, 543–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, D.K.; Lane, M. Alleviation of the “2-cell block” and development to the blastocyst of CF1 mouse embryos: Role of amino acids, EDTA and physical parameters. Hum. Reprod. 1996, 11, 2703–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, D.K. Changes in requirements and utilization of nutrients during mammalian preimplantation development and their significance in embryo culture. Theriogenology 1998, 49, 83–102. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Culture and selection of viable blastocysts: A feasible proposition for human IVF? Hum. Reprod. Update 1997, 3, 367–382. [Google Scholar] [CrossRef]
- Gardner, D.K.; Vella, P.; Lane, M.; Wagley, L.; Schlenker, T.; Schoolcraft, W.B. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil. Steril. 1998, 69, 84–88. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Calderon, I.; Leeton, J. Environment of the preimplantation embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of the cumulus cells. Fertil. Steril. 1996, 65, 349–353. [Google Scholar] [CrossRef]
- Wales, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2015, 22, 2–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, M.; Gardner, D.K. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum. Reprod. 1992, 77, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.L.; Woodward, B.J.; Swain, J.E. Single or group culture of mammalian embryos: The verdict of the literature. J. Reprod. Stem Cell Biotechnol. 2011, 22, 77–87. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Amino acids and ammonium production regulates mouse embryo development in culture. Biol. Reprod. 1993, 48, 377–385. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol. Reprod. 2003, 69, 1109–1117. [Google Scholar] [CrossRef]
- Otsuki, J.; Nagai, Y.; Chiba, K. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil. Steril. 2008, 91, 1745–1749. [Google Scholar] [CrossRef]
- Otsuki, J.; Nagai, Y.; Chiba, K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil. Steril. 2007, 88, 741–743. [Google Scholar] [CrossRef]
- Tae, J.C.; Kim, E.Y.; Lee, W.D.; Park, S.P.; Lim, J.H. Sterile filtered paraffin oil supports in vitro developmental competence in bovine embryos comparable to co-culture. J. Assist. Reprod. Genet. 2006, 23, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.K.; Lane, M. Development of viable mammalian embryos in vitro: Evolution of sequential media. In Principles of Cloning; Cibelli, J., Campbell, K.H.S., Lanza, R.P., West, M.D., Eds.; Academic Press: Cambridge, UK, 2002; pp. 187–213. [Google Scholar]
- Pool, T.B. An update on embryo culture for assisted reproductive technologies: Performance and safety. Semin. Reprod. Med. 2005, 23, 309–318. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Embryo culture medium: Which is the best? Best Prac. Res. Clin. Ob. Gyn. 2007, 21, 83–100. [Google Scholar] [CrossRef]
- Summers, M.C. A brief history of the development of the KSOM family of media. J. Assist. Reprod. Genet. 2013, 30, 995–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggers, J.D.; McGinnis, I.K.; Lawitts, J.A. Enhanced effect of glycyl-L-glutamine on mouse preimplantation embryos in vitro. Reprod. Biomed. Online. 2004, 9, 59–69. [Google Scholar] [CrossRef]
- Summers, M.C.; McGinnis, L.K.; Lawitts, J.A.; Biggers, J.D. Mouse embryo development following IVF in media containing either L-glutamine or glycyl-L-glutamine. Hum. Reprod. 2005, 20, 1364–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Rodriegez-Martinez, H.; Lane, M. Fetal development after transfer is increased by replacing protein with glycoaminoglycan hyaluronan for mouse embryo culture and transfer. Hum. Reprod. 1999, 14, 2575–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, K.; Miyano, T.; Kato, S. Effect of glycoaminoglycans on the development of in vitro-matured and fertilized porcine oocytes to the blastocyst stage in vitro. Biol. Reprod. 1998, 58, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Urman, B.; Yakin, K.; Isiklar, A.; Balaban, B. Effect of hyaluronan-enriched transfer medium on implantation and pregnancy rates after day 3 and day 5 embryo transfers: A prospective randomized study. Fertil. Steril. 2008, 90, 604–612. [Google Scholar] [CrossRef]
- Gordon, C.; Whitney, J.B.; Hatch, I.; Nugent, N.; Zozula, S.; Anderson, R.E.; Schiewe, M.C. Patients of advanced maternal age should only transfer a single euploid blastocyst. J. Reprod. Endocrinol. Infertil. 2018, 44, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Whitney, J.B.; Schiewe, M.C.; Anderson, R.E. Single center validation of routine blastocyst biopsy implementation. J. Asst. Reprod. Genet. 2016, 33, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Schiewe, M.C. An effective, simplified and practical approach to intracytoplasmic sperm injection at multiple IVF Centers. J. Asst. Reprod. Genet. 1996, 13, 238–245. [Google Scholar] [CrossRef]
- Schiewe, M.C.; Whitney, J.B.; Anderson, R.E. Potential risk of monochorionic dizygotic twin blastocyst formation associated with early laser zona dissection of group cultured embryos. Fertil. Steril. 2015, 103, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Schiewe, M.C.; Zozula, S.; Anderson, R.E.; Fahy, G.M. Validation of microSecure vitrification (mS-VTF) for the effective cryopreservation of human embryos and oocytes. Cryobiol. 2015, 71, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiewe, M.C.; Zozula, S.; Nugent, N.; Waggoner, K.; Borba, J.; Whitney, J.B. Modified microSecure vitrification: A safe, simple and highly effective cryopreservation procedure for human blastocysts. J. Vis. Exp. 2017, 121, e54871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.E.; Nugent, N.L.; Gregg, A.T.; Nunn, S.L.; Behr, B.R. Transvaginal ultrasound guided embryo transfer (TVGET): A novel technique, which improves outcome in patients with previous failed IVF cycles. Fertil. Steril. 2002, 77, 769–775. [Google Scholar] [CrossRef]
- Anderson, R.E.; Whitney, J.B.; Schiewe, M.C. Clinical benefits of preimplantation genetic testing for aneuploidy (PGT-A) for all in vitro fertilization treatment cycles. Eur. J. Med. Genet. 2019, 63, 103731, In press (online 27 July 2020). [Google Scholar] [CrossRef] [PubMed]
- Biggers, J.D.; Racowsky, C. The development of fertilized human ova to the blastocyst stage in KSOM (AA) medium: Is a two-step protocol necessary? Reprod. Biomed. Online 2002, 5, 133–140. [Google Scholar] [CrossRef]
- Hardarson, T.; Bungum, M.; Conaghan, J.; Meintjes, M.; Chantilis, S.J.; Molnar, L.; Gunnarsson, K.; Wikland, M. Noninferiority, randomized, controlled trial comparing embryo development using media developed for sequential or undisturbed culture in a time-lapse set-up. Fertil. Steril. 2015, 104, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Costa-Borges, N.; Belles, M.; Meseguer, M.; Galliano, D.; Ballesteros, A.; Calderón, G. Blastocyst development in single medium with or without renewal on day 3: A prospective cohort study on sibling donor oocytes in a time-lapse incubator. Fertil. Steril. 2016, 105, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Biggers, J.D.; Summers, M.C. Choosing a culture medium: Making informed choices. Fertil. Steril. 2008, 90, 473–483. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Toward a single embryo transfer. RBM Online. 2003, 66, 470–481. [Google Scholar] [CrossRef]
- Catt, J.W.; Henman, M. Toxic effects of oxygen on human embryo development in vitro. Hum. Reprod. 2000, 15, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fert. 1993, 99, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Batt, P.A.; Gardner, D.K.; Cameron, A.W. Oxygen concentration and protein source affect the development of preimplantation goat embryos in vitro. Reprod. Fertil. Dev. 1991, 3, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Meintjes, M.; Chantilis, S.J.; Douglas, J.D.; Rodriguez, A.J.; Guerami, A.R.; Bookout, D.M.; Barnett, B.D.; Madden, J.D. A controlled randomized trial evaluating the effect of lowered oxygen tension on live births in a predominantly blastocyst transfer program. Hum. Reprod. 2009, 24, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.K.; Surrey, E.; Minjarez, D.; Leitz, A.; Stevens, J.; Schoolcraft, W.B. Single blastocyst transfer: A prospective randomized trial. Ferti. Steril. 2004, 81, 551–555. [Google Scholar] [CrossRef]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996, 74, A1–A7. [Google Scholar] [CrossRef]
- Gardner, D.K. Lactate production by the mammalian blastocyst: Manipulating the microenvironment for uterine implantation and invasion? Bioessays 2015, 37, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Bontekoe, S.; Heineman, M.J.; Johnson, N.; Blake, D. Adherence compounds in embryo transfer media for assisted reproductive technologies. Cochrane Database Syst. Rev. 2014, 25, CD007421. [Google Scholar] [CrossRef] [Green Version]
- Pool, T.B.; Martin, J.E. High continuing pregnancy rates after in vitro fertilization-embryo transfer using medium supplemented with a plasma protein fraction* containing α- and β-globulins. Fertil. Steril. 1994, 61, 714–719. [Google Scholar] [CrossRef]
- Weathersbee, P.; Pool, T.B.; Ord, T. Synthetic serum substitute (SSS): A globulin-enriched protein supplement for human embryo culture. J. Asst. Reprod. Genet. 1995, 12, 354–360. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod. Fertil. Dev. 2005, 17, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod. Fertil. Dev. 2005, 17, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Pool, T.B.; Schoolfield, J.; Han, D. Human embryo culture media comparisons. Methods Mol. Biol. 2012, 912, 367–386. [Google Scholar] [CrossRef] [PubMed]
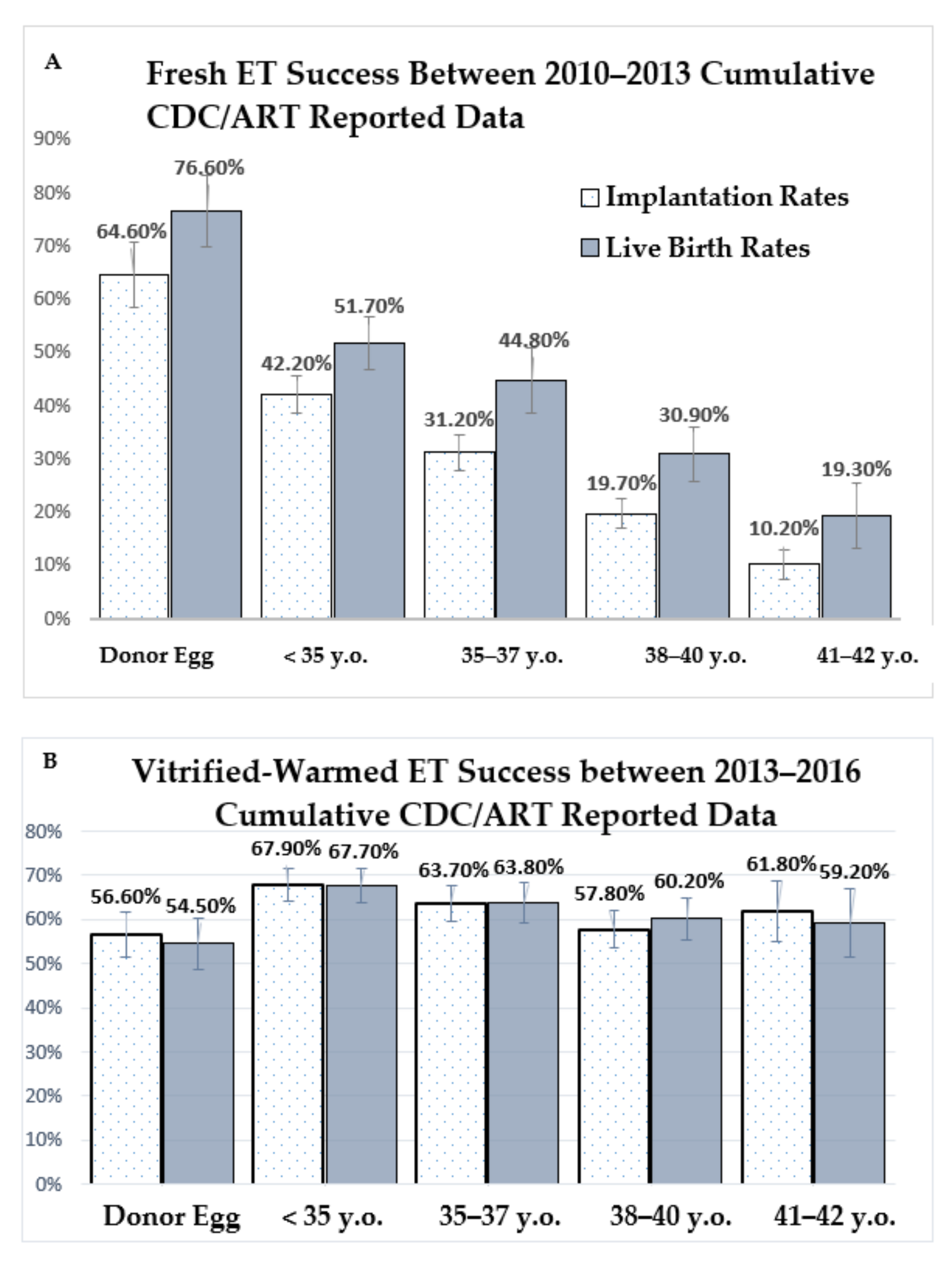
| AGE <35 yo + Donor Cycles | AGE <38 yo + Donors | |||||
|---|---|---|---|---|---|---|
| Categories | n δ | # (%)+β-hCG | # (%) Live Birth | n | # (%)+ß-hCG | # (%) Live Birth |
| 6% CO2/Air Incubation | ||||||
| [2ndQrt06] | ||||||
| P1/G1.3/2.3 media | 49 (7) δ | 40 (82) * | 31 (63) * | 75 | 54 (72) | 42 (56) |
| [4thQrt06] | ||||||
| mLG medium ** | 49 (5) δ | 29 (59) | 24 (49) | 65 | 42 (65) | 33 (51) |
| 6% CO2/5% O2/89% N2 Incubation {mLG media} | ||||||
| [1stQrt07] | ||||||
| Sanyo Mini | 29 (4) δ | 22 (76) | 21 (72) * | 38 | 25 (66) | 24 (63) |
| Heraeus 240 | 21 (3) δ | 15 (71) | 13 (62) | 32 | 24 (75) * | 20 (63) |
| Combined TG | 50 (7) δ | 37 (74) | 34 (68) | 70 | 49 (70) | 44 (63) |
| Day 3 | Day 5/6 | Mean | |||||
|---|---|---|---|---|---|---|---|
| Group | INC | N | 8–10 cell/A | BL/AA-BB * | # ET | # (%) + β-hCG | # (%) Live Birth |
| Donor Egg | TG | 29 | 62.80% | 60.30% | 2.28 | 25 (86.2%) | 22 (75.9%) |
| CO2 | 17 | 72.30% | 14.50% | 2.65 | 13 (77%) | 11 (65.0%) | |
| ≤ 34 yo | TG | 69 | 64.90% | 53.90% | 2.63 | 53 (76.8%) | 50 (72.5%) |
| CO2 | 65 | 65.70% | 31.50% | 3.03 | 47 (72.3%) | 38 (58.5%) | |
| 35–37 yo | TG | 38 | 59.70% | 47.00% | 2.95 | 25 (65.8%) | 23 (60.5%) |
| CO2 | 47 | 69.30% | 15% | 3.77 | 23 (49%) | 19 (40.4%) | |
| 38–40 yo | TG | 49 | 65.70% | 51.40% | 3.39 | 24 (46.9%) | 21 (42.9%) |
| CO2 | 17 | 82.90% | 15.80% | 3.96 | 18 (35.3%) | 11 (21.6%) | |
| 41–43 yo | TG | 41 | 60.00% | 25.50% | 2.8 | 13 (31.7%) | 7 (17.1%) |
| CO2 | 17 | 60.00% | 13.70% | 4.03 | 9 (29%) | 6 (19.4%) |
| Pt. Age, ET | #Pts | µ #/ET | +b-hCG (%) | Clinical Preg. (%) | Live Birth (%) | # BL AA-BB (%) |
|---|---|---|---|---|---|---|
| Donor, D5 | 53 | 2.2 | 41 (77) | 38 (72) | 36 (68) * | 469/711 (66) |
| ≤34yo, D5 | 88 | 2.1 | 64 (73) * | 57 (65) * | 48 (55) * | 602/1039 (58) |
| 35–37yo, D5 | 49 | 2.5 | 34 (69) * | 32 (65) * | 29 (59) * | 312/529 (59) |
| 38–40yo, D5 | 40 | 3.1 | 28 (70) * | 21 (53) * | 17 (43) * | 216/423 (51) |
| 41–43yo, D5 | 12 | 4.0 | 9 (75) * | 7 (58) * | 3 (25) * | 57/101 (56) |
| Donor, D3 | 16 | 2.7 | 13 (81) | 12 (75) | 9 (56) | ND |
| ≤ 34yo, D3 | 61 | 3.2 | 35 (57) | 33 (54) | 26 (43) | ND |
| 35–37yo, D3 | 63 | 3.4 | 32 (51) | 29 (46) | 22 (35) | ND |
| 38–40yo, D3 | 76 | 3.6 | 43 (57) | 33 (43) | 19 (25) | ND |
| 41–43yo, D3 | 66 | 3.3 | 16 (24) | 14 (21) | 6 (9) | ND |
| Experiment A | ||||||
| Age/Treatment | #Pts | #/BLET | +β-hCG (%) | Clinical Preg. (%) | Live Birth (%) | #BL AA-BB (%) * |
| ≤34, G1/2.5 | 26 | 2.1 | 20 (77) | 20 (77) a | 19 (73) a | 142/285 (50) a |
| ≤34, mLG | 24 | 2 | 17 (71) | 13 (54) b | 11 (46) b | 149/241 (62) b |
| 35–40, G1/2.5 | 17 | 2.4 | 11 (65) | 9 (53) | 8 (47) | 58/149 (39) |
| 35–40, mLG | 23 | 2.4 | 16 (70) | 13 (57) | 11 (48) | 86/203 (42) |
| Experiment B | ||||||
| ≤34, mLg, + Ovoil™ | 33 | 2 | 27 (82) | 24 (73) | 22 (67) | 297/418 (71) a |
| 35–40 mLG, + Ovoil™ | 28 | 2.6 | 20 (71) | 19 (68) | 16 (57) | 95/162 (60) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiewe, M.C.; Zozula, S.; Nugent, N.L.; Whitney, J.B.; Hatch, I.; Lee, C.T.; Anderson, R.E. Systematic Development, Validation and Optimization of a Human Embryo Culture System. Reprod. Med. 2020, 1, 1-14. https://doi.org/10.3390/reprodmed1010001
Schiewe MC, Zozula S, Nugent NL, Whitney JB, Hatch I, Lee CT, Anderson RE. Systematic Development, Validation and Optimization of a Human Embryo Culture System. Reproductive Medicine. 2020; 1(1):1-14. https://doi.org/10.3390/reprodmed1010001
Chicago/Turabian StyleSchiewe, Mitchel C., Shane Zozula, Nancy L. Nugent, John B. Whitney, Ilene Hatch, C. Terence Lee, and Robert E. Anderson. 2020. "Systematic Development, Validation and Optimization of a Human Embryo Culture System" Reproductive Medicine 1, no. 1: 1-14. https://doi.org/10.3390/reprodmed1010001




